Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity
- PMID: 10430952
- PMCID: PMC17792
- DOI: 10.1073/pnas.96.16.9385
Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity
Abstract
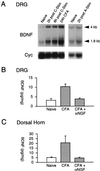
Brain-derived neurotrophic factor (BDNF) is expressed in nociceptive sensory neurons and transported anterogradely to the dorsal horn of the spinal cord where it is located in dense core vesicles in C-fiber terminals. Peripheral inflammation substantially up-regulates BDNF mRNA and protein in the dorsal root ganglion (DRG) in a nerve growth factor-dependent fashion and results in novel expression of BDNF by DRG neurons with myelinated axons. C-fiber electrical activity also increases BDNF expression in the DRG, and both inflammation and activity increase full-length TrkB receptor levels in the dorsal horn. Sequestration of endogenous BDNF/neurotrophin 4 by intraspinal TrkB-Fc fusion protein administration does not, in noninflamed animals, change basal pain sensitivity nor the mechanical hypersensitivity induced by peripheral capsaicin administration, a measure of C fiber-mediated central sensitization. TrkB-Fc administration also does not modify basal inflammatory pain hypersensitivity, but does block the progressive hypersensitivity elicited by low-intensity tactile stimulation of inflamed tissue. BDNF, by virtue of its nerve growth factor regulation in sensory neurons including novel expression in A fibers, has a role as a central modulator of tactile stimulus-induced inflammatory pain hypersensitivity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

