ITM2A is induced during thymocyte selection and T cell activation and causes downregulation of CD8 when overexpressed in CD4(+)CD8(+) double positive thymocytes
- PMID: 10432285
- PMCID: PMC2195576
- DOI: 10.1084/jem.190.2.217
ITM2A is induced during thymocyte selection and T cell activation and causes downregulation of CD8 when overexpressed in CD4(+)CD8(+) double positive thymocytes
Abstract
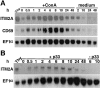
To identify novel genes that are involved in positive selection of thymocytes, we performed polymerase chain reaction (PCR)-based subtractive hybridization between selecting and nonselecting thymi. OT-1 T cell receptor (TCR) transgenic thymocytes on a recombination activating gene (RAG) null background are efficiently selected into the CD8 lineage in H-2(b) mice (RAG-2(-/-)OT-1, selecting thymi), but are not selected on a transporter associated with antigen processing (TAP) null background (RAG-2(-/-)TAP-1(-/-)OT-1, nonselecting thymi). We report here our studies of one gene, ITM2A, whose expression is dramatically higher in T cells in the selecting thymus. The expression pattern of ITM2A in thymocyte subsets correlates with upregulation during positive selection. In addition, ITM2A expression is higher in the thymus than in either the spleen or lymph nodes, but can be upregulated in peripheral T cells upon activation. ITM2A expression was also induced in RAG-2(-/-) thymocytes in vivo upon CD3 cross-linking. We demonstrate that ITM2A is a type II membrane glycoprotein that exists as two species with apparent M(r) of 45 and 43 kD and appears to localize primarily to large cytoplasmic vesicles and the Golgi apparatus, but is also expressed on the cell surface. Expression on the surface of EL4 cells increases with activation by phorbol myristate acetate (PMA) and ionomycin. Finally, overexpression of ITM2A under control of the lck proximal promoter in mice results in partial downregulation of CD8 in CD4(+)CD8(+) double positive (DP) thymocytes, and a corresponding increase in the number of CD4(+)CD8(lo) thymocytes. Possible roles for this novel activation marker in thymocyte development are discussed.
Figures








References
-
- Kisielow P., von Boehmer H. Development and selection of T cellsfacts and puzzles. Adv. Immunol. 1995;58:87–209. - PubMed
-
- Jameson S.C., Bevan M.J. T-cell selection. Curr. Opin. Immunol. 1998;10:214–219. - PubMed
-
- Brandle D., Muller S., Muller C., Hengartner H., Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur. J. Immunol. 1994;24:145–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

