Block of rapid depolarization induced by in vitro energy depletion of rat dorsal vagal motoneurones
- PMID: 10432344
- PMCID: PMC2269489
- DOI: 10.1111/j.1469-7793.1999.0131o.x
Block of rapid depolarization induced by in vitro energy depletion of rat dorsal vagal motoneurones
Abstract
1. The ionic mechanisms contributing to the rapid depolarization (RD) induced by in vitro ischaemia have been studied in dorsal vagal motoneurones (DVMs) of brainstem slices. Compared with CA1 hippocampal neurones, RD of DVMs was slower, generally occurred from a more depolarized membrane potential and was accompanied by smaller increases in [K+]o. 2. RD was not induced by elevation of [K+]o to values measured around DVMs during in vitro ischaemia or by a combination of raised [K+]o and 2-5 microM ouabain. 3. Neither TTX (5-10 microM) nor TTX combined with bepridil (10-30 microM), a Na+-Ca2+ exchange inhibitor, slowed RD. Block of voltage-dependent Ca2+ channels with Cd2+ (0.2 mM) and Ni2+ (0.3 mM) led to an earlier onset of RD, possibly because [K+]o was higher than that measured during in vitro ischaemia in the absence of divalent ions. 4. When [Na+]o was reduced to 11.25-25 mM, RD did not occur, although a slow depolarization was observed. RD was slowed (i) by 10 mM Mg2+ and 0.5 mM Ca2+, (ii) by a combination of TTX (1.5-5 microM), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 microM) and D-2-amino-5-phosphonovalerate (AP5, 50 microM) and (iii) by TTX (1.5-5 microM) and AP5 (50 microM). 5. Ni2+ at concentrations of 0.6 or 1.33 mM blocked RD whereas 0.6 mM Cd2+ did not. A combination of Cd2+ (0.2 mM), Ni2+ (0.3 mM), AP5 (50 microM) and bepridil (10 microM) was largely able to mimic the effects of high concentrations of Ni2+. 6. It is concluded that RD is due to Na+ entry, predominantly through N-methyl-D-aspartate receptor ionophores, and to Ca2+ entry through voltage-dependent Ca2+ channels. These results are consistent with known changes in the concentrations of extracellular ions when ischaemia-induced rapid depolarization occurs.
Figures
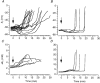


 ) at time zero (arrow; see Methods). B, effects on membrane potential of 2–5 μM ouabain when [K+]o was raised to 10 mM. C and D, changes in membrane potential (C) and contralaterally measured extracellular K+ activity (D) induced by 25 μM ouabain.
) at time zero (arrow; see Methods). B, effects on membrane potential of 2–5 μM ouabain when [K+]o was raised to 10 mM. C and D, changes in membrane potential (C) and contralaterally measured extracellular K+ activity (D) induced by 25 μM ouabain.


 ) (A); when the extracellular Na+ concentration was reduced to 11.25 (
) (A); when the extracellular Na+ concentration was reduced to 11.25 ( ), 18 (−) or 25 mM (
), 18 (−) or 25 mM ( ) (B); in the presence of 3–5 μM TTX and 50 μM AP5, with (−) and without 10 μM CNQX (
) (B); in the presence of 3–5 μM TTX and 50 μM AP5, with (−) and without 10 μM CNQX ( ) (C). Perfusate switched at time zero (arrow; see Methods).
) (C). Perfusate switched at time zero (arrow; see Methods).
 ) or 1.33 mM Ni2+ (−) (B); 0.3 mM Ni2+ (
) or 1.33 mM Ni2+ (−) (B); 0.3 mM Ni2+ ( ) or 0.6 mM Cd2+ (−) (C); 0.2 mM Cd2+, 0.3 mM Ni2+, 50 μM AP5 and 10 μM bepridil, with (−) and without (
) or 0.6 mM Cd2+ (−) (C); 0.2 mM Cd2+, 0.3 mM Ni2+, 50 μM AP5 and 10 μM bepridil, with (−) and without ( ) 3–5 μM TTX (D). Perfusate switched at time zero (arrow; see Methods).
) 3–5 μM TTX (D). Perfusate switched at time zero (arrow; see Methods).References
-
- Aitken PG, Balestrino M, Somjen GG. NMDA antagonists: lack of protective effect against hypoxic damage in CA1 region of hippocampal slices. Neuroscience Letters. 1988;89:187–192. - PubMed
-
- Aitken PG, Jing J, Young J, Somjen GG. Ion channel involvement in hypoxia-induced spreading depression in hippocampal slices. Brain Research. 1991;541:7–11. - PubMed
-
- Costa PF, Ribeiro MA, Santos AI. Afterpotential characteristics and firing patterns in maturing rat hippocampal CA1 neurones in in vitro slices. Developmental Brain Research. 1991;62:263–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

