Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes
- PMID: 10432346
- PMCID: PMC2269481
- DOI: 10.1111/j.1469-7793.1999.0153o.x
Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes
Abstract
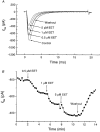
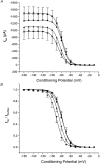
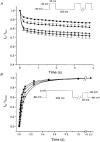
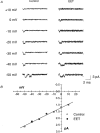
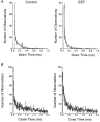
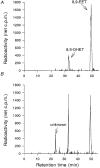
1. Whole-cell Na+ currents (holding potential, -80 mV; test potential, -30 mV) in rat myocytes were inhibited by 8, 9-epoxyeicosatrienoic acid (8,9-EET) in a dose-dependent manner with 22+/-4% inhibition at 0.5 microM, 48+/-5% at 1 microM, and 73+/-5% at 5 microM (mean +/- S.E.M., n = 10, P<0.05 for each dose vs. control). Similar results were obtained with 5,6-, 11,12-, and 14,15-EETs, while 8,9-dihydroxyeicosatrienoic acid (DHET) was 3-fold less potent and arachidonic acid was 10- to 20-fold less potent. 2. 8,9-EET produced a dose-dependent, hyperpolarized shift in the steady-state membrane potential at half-maximum inactivation (V ), without changing the slope factor. 8,9-EET had no effect on the steady-state activation of Na+ currents. 3. Inhibition of Na+ currents by 8,9-EET was use dependent, and channel recovery was slowed. The effects of 8,9-EET were greater at depolarized potentials. 4. Single channel recordings showed 8,9-EET did not change the conductance or the number of active Na+ channels, but markedly decreased the probability of Na+ channel opening. These results were associated with a decrease in the channel open time and an increase in the channel closed times. 5. Incubation of cultured cardiac myocytes with 1 microM [3H]8,9-EET showed that 25% of the radioactivity was taken up by the cells over a 2 h period, and most of the uptake was incorporated into phospholipids, principally phosphatidylcholine. Analysis of the medium after a 2 h incubation indicated that 86% of the radioactivity remained as [3H]8,9-EET while 13% was converted into [3H]8,9-DHET. After a 30 min incubation, 1-2% of the [3H]8,9-EET uptake by cells remained as unesterified EET. 6. These results demonstrate that cardiac cells have a high capacity to take up and metabolize 8,9-EET. 8,9-EET is a potent use- and voltage-dependent inhibitor of the cardiac Na+ channels through modulation of the channel gating behaviour.
Figures




References
-
- Abraham NG, Pinto A, Mullane K. Identification of heme oxygenase and cytochrome P-450 in the rabbit heart. Journal of Molecular and Cellular Cardiology. 1987;19:73–81. - PubMed
-
- Atkins DL, Rosenthal JK, Krumm PA, Marvin WJ., Jr Differential growth of neonatal WYK and SHR ventricular myocytes within sympathetic co-cultures. Journal of Molecular and Cellular Cardiology. 1992;24:1479–1489. - PubMed
-
- Bendahhou S, Cummins TR, Agnew WS. Mechanism of modulation of the voltage-gated skeletal and cardiac muscle sodium channels by fatty acids. American Journal of Physiology. 1997;272:C592–600. - PubMed
-
- Benndorf K. Low-noise recording. In: Sakman B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. pp. 129–145.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

