Role of N-type calcium channels in autonomic neurotransmission in guinea-pig isolated left atria
- PMID: 10433500
- PMCID: PMC1566099
- DOI: 10.1038/sj.bjp.0702629
Role of N-type calcium channels in autonomic neurotransmission in guinea-pig isolated left atria
Abstract
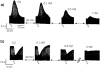
1. Calcium entry via neuronal calcium channels is essential for the process of neurotransmission. We investigated the calcium channel subtypes involved in the operation of cardiac autonomic neurotransmission by examining the effects of selective calcium channel blockers on the inotropic responses to electrical field stimulation (EFS) of driven (4 Hz) guinea-pig isolated left atria. In this tissue, a previous report (Hong & Chang, 1995) found no evidence for N-type channels involved in the vagal negative inotropic response and only weak involvement in sympathetic responses. 2. The effects of cumulative concentrations of the selective N-type calcium channel blocker, omega-conotoxin GVIA (GVIA; 0.1-10 nM) and the non-selective N-, P/Q-type calcium channel blocker, omega-conotoxin MVIIC (MVIIC; 0.01-10 nM) were examined on the positive (with atropine, 1 microM present) and negative (with propranolol, 1 microM and clonidine, 1 microM present) inotropic responses to EFS (eight trains, each train four pulses per punctate stimulus). 3. GVIA caused complete inhibition of both cardiac vagal and sympathetic inotropic responses to EFS. GVIA was equipotent at inhibiting positive (pIC50 9.29+/-0.08) and negative (pIC50 9.13+/-0.17) inotropic responses. MVIIC also mediated complete inhibition of inotropic responses to EFS and was 160 and 85 fold less potent than GVIA at inhibiting positive (pIC50 7.08+/-0.10) and negative (pIC50 7.20+/-0.14) inotropic responses, respectively. MVIIC was also equipotent at inhibiting both sympathetic and vagal responses. 4. Our data demonstrates that N-type calcium channels account for all the calcium current required for cardiac autonomic neurotransmission in the guinea-pig isolated left atrium.
Figures
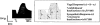



Similar articles
-
Calcium channel subtypes for the sympathetic and parasympathetic nerves of guinea-pig atria.Br J Pharmacol. 1995 Sep;116(1):1577-82. doi: 10.1111/j.1476-5381.1995.tb16375.x. Br J Pharmacol. 1995. PMID: 8564221 Free PMC article.
-
Effects of N-, P- and Q-type neuronal calcium channel antagonists on mammalian peripheral neurotransmission.Br J Pharmacol. 1996 Sep;119(1):49-56. doi: 10.1111/j.1476-5381.1996.tb15676.x. Br J Pharmacol. 1996. PMID: 8872356 Free PMC article.
-
Effects of omega-toxins on noradrenergic neurotransmission in beating guinea pig atria.Eur J Pharmacol. 1995 Apr 4;276(3):231-8. doi: 10.1016/0014-2999(95)00032-g. Eur J Pharmacol. 1995. PMID: 7601208
-
Neuropeptide Y is a prejunctional inhibitor of vagal but not sympathetic inotropic responses in guinea-pig isolated left atria.Br J Pharmacol. 1999 May;127(2):383-90. doi: 10.1038/sj.bjp.0702565. Br J Pharmacol. 1999. PMID: 10385237 Free PMC article.
-
Calcium channel subtypes for cholinergic and nonadrenergic noncholinergic neurotransmission in isolated guinea pig trachea.Naunyn Schmiedebergs Arch Pharmacol. 2010 Dec;382(5-6):419-32. doi: 10.1007/s00210-010-0556-z. Epub 2010 Sep 5. Naunyn Schmiedebergs Arch Pharmacol. 2010. PMID: 20820758
Cited by
-
Differential role of N-type calcium channel splice isoforms in pain.J Neurosci. 2007 Jun 13;27(24):6363-73. doi: 10.1523/JNEUROSCI.0307-07.2007. J Neurosci. 2007. PMID: 17567797 Free PMC article.
-
Functional disorders of the sympathetic nervous system in mice lacking the alpha 1B subunit (Cav 2.2) of N-type calcium channels.Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5323-8. doi: 10.1073/pnas.081089398. Epub 2001 Apr 10. Proc Natl Acad Sci U S A. 2001. PMID: 11296258 Free PMC article.
-
Tumour necrosis factor alpha activates nuclear factor kappaB signalling to reduce N-type voltage-gated Ca2+ current in postganglionic sympathetic neurons.J Physiol. 2009 Jun 1;587(Pt 11):2623-34. doi: 10.1113/jphysiol.2009.172312. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403618 Free PMC article.
References
-
- ADAMS M.E., MYERS R.A., IMPERIAL J.S., OLIVERA B.M. Toxityping rat brain calcium channels with ω-toxins from spider and cone snail venoms. Biochemistry. 1993;32:12566–12570. - PubMed
-
- ANGUS J.A., HARVEY K. Refractory period field stimulation of right atria: A method for studying presynaptic receptors in cardiac autonomic transmission. J. Pharmacol. Meth. 1981;6:51–64. - PubMed
-
- BOOT J.R. Differential effects of ω-conotoxin GVIA and MVIIC on nerve stimulation induced contractions of guinea-pig ileum and rat vas deferens. Eur. J. Pharmacol. 1994;258:155–158. - PubMed
-
- DUNLAP K., LUEBKE J.I., TURNER T.J. Identification of calcium currents that control neurosecretion. Science. 1994;266:828–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

