Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targetting of NF-kappaB and lack of I-kappaB involvement
- PMID: 10433509
- PMCID: PMC1566089
- DOI: 10.1038/sj.bjp.0702613
Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targetting of NF-kappaB and lack of I-kappaB involvement
Retraction in
-
RETRACTION: Ligand-Induced Differentiation of Glucocorticoid Receptor (GR) Trans-Repression and Transactivation: Preferential Targeting of NF-κB and Lack of I-κB Involvement.Br J Pharmacol. 2025 Dec 8. doi: 10.1111/bph.70309. Online ahead of print. Br J Pharmacol. 2025. PMID: 41360625 No abstract available.
Abstract
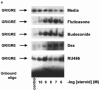
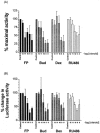
1. Glucocorticoids are highly effective in controlling chronic inflammatory diseases, such as asthma and rheumatoid arthritis, but the exact molecular mechanism of their anti-inflammatory action remains uncertain. They act by binding to a cytosolic receptor (GR) resulting in activation or repression of gene expression. This may occur via direct binding of the GR to DNA (transactivation) or by inhibition of the activity of transcription factors such as AP-1 and NF-kappaB (transrepression). 2. The topically active steroids fluticasone propionate (EC50= 1.8 x 10(-11) M) and budesonide (EC50=5.0 x 10(-11) M) were more potent in inhibiting GM-CSF release from A549 cells than tipredane (EC50 = 8.3 x 10(-10)) M), butixicort (EC50 = 3.7 x 10(-8) M) and dexamethasone (EC50 = 2.2 x 10(-9) M). The anti-glucocorticoid RU486 also inhibited GM-CSF release in these cells (IC50= 1.8 x 10(-10) M). 3. The concentration-dependent ability of fluticasone propionate (EC50 = 9.8 x 10(-10) M), budesonide (EC50= 1.1 x 10(-9) M) and dexamethasone (EC50 = 3.6 x 10(-8) M) to induce transcription of the beta2-receptor was found to correlate with GR DNA binding and occurred at 10-100 fold higher concentrations than the inhibition of GM-CSF release. No induction of the endogenous inhibitors of NF-kappaB, IkappaBalpha or I-kappaBbeta, was seen at 24 h and the ability of IL-1beta to degrade and subsequently induce IkappaBalpha was not altered by glucocorticoids. 4. The ability of fluticasone propionate (IC50=0.5 x 10(-11) M), budesonide (IC50=2.7 x 10(-11) M), dexamethasone (IC50=0.5 x 10(-9) M) and RU486 (IC50=2.7 x 10(-11) M) to inhibit a 3 x kappaB was associated with inhibition of GM-CSF release. 5. These data suggest that the anti-inflammatory properties of a range of glucocorticoids relate to their ability to transrepress rather than transactivate genes.
Figures
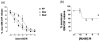
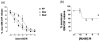








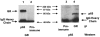
References
-
- ADCOCK I.M., GILBEY T., GELDER C.M., CHUNG K.F., BARNES P.J. Glucocorticoid receptor localization in normal and asthmatic lung. Am. J. Respir. Crit. Care Med. 1996;154:771–782. - PubMed
-
- ADCOCK I.M., LANE S.J., BROWN C.R., PETERS M.J., LEE T.H., BARNES P.J. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J. Immunol. 1995;154:3500–3505. - PubMed
-
- AUPHAN N., DIDONATO J.A., ROSETTE C., HELMBERG A., KARIN M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. - PubMed
-
- BARNES P.J. Inhaled glucocorticoids for asthma. N. Engl. J. Med. 1995;332:868–875. - PubMed
-
- BARNES P.J., ADCOCK I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends. Pharmacol. Sci. 1993;14:436–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

