A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain
- PMID: 10433933
- PMCID: PMC1866852
- DOI: 10.1016/S0002-9440(10)65136-X
A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain
Abstract
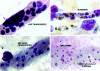
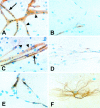
Microvascular sequestration was assessed in the brains of 50 Thai and Vietnamese patients who died from severe malaria (Plasmodium falciparum, 49; P. vivax, 1). Malaria parasites were sequestered in 46 cases; in 3 intravascular malaria pigment but no parasites were evident; and in the P. vivax case there was no sequestration. Cerebrovascular endothelial expression of the putative cytoadherence receptors ICAM-1, VCAM-1, E-selectin, and chondroitin sulfate and also HLA class II was increased. The median (range) ratio of cerebral to peripheral blood parasitemia was 40 (1.8 to 1500). Within the same brain different vessels had discrete but different populations of parasites, indicating that the adhesion characteristics of cerebrovascular endothelium change asynchronously during malaria and also that significant recirculation of parasitized erythrocytes following sequestration is unlikely. The median (range) ratio of schizonts to trophozoites (0.15:1; 0.0 to 11.7) was significantly lower than predicted from the parasite life cycle (P < 0.001). Antimalarial treatment arrests development at the trophozoite stages which remain sequestered in the brain. There were significantly more ring form parasites (age < 26 hours) in the cerebral microvasculature (median range: 19%; 0-90%) than expected from free mixing of these cells in the systemic circulation (median range ring parasitemia: 1.8%; 0-36.2%). All developmental stages of P. falciparum are sequestered in the brain in severe malaria.
Figures






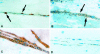

References
-
- White NJ, Ho M: The pathophysiology of malaria. Adv Parasitol 1992, 31:83-173 - PubMed
-
- Clark IA, Rockett KA, Cowden WB: Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet 1992, 340:894-896 - PubMed
-
- Clark IA, Rockett KA: The cytokine theory of cerebral malaria. Parasitol Today 1994, 10:410-412 - PubMed
-
- Berendt AR, Turner GDH, Newbold CI: Cerebral malaria: the sequestration hypothesis. Parasitol Today 1994, 10:412-414 - PubMed
-
- Udeinnya IJ, Schmidt JA, Aikawa M, Miller LM, Green I: Falciparum malaria infected erythrocytes specifically bind to cultured human endothelial cells. Science 1981, 213:555-557 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

