Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas
- PMID: 10433937
- PMCID: PMC1866855
- DOI: 10.1016/S0002-9440(10)65140-1
Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas
Abstract
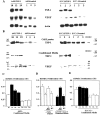
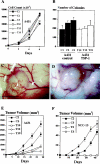
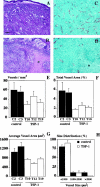
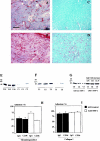
The function of the endogenous angiogenesis inhibitor thrombospondin-1 (TSP-1) in epithelial tumor development has remained controversial. We studied the in vitro growth characteristics and the in vivo tumor xenograft growth of the human squamous cell carcinoma cell lines A431 and SCC-13, stably transfected to overexpress human TSP-1. Overexpression of TSP-1 inhibited tumor growth of A431 xenotransplants, and completely abolished tumor formation by SCC-13 cells. TSP-1 overexpressing A431 tumors were characterized by extensive areas of necrosis and by decreased tumor vessel number and size. The effects of TSP-1 on tumor cell growth were indirect since tumor cell proliferation rates in vivo and in vitro, anchorage-dependent and -independent growth in vitro, and susceptibility to induction of apoptosis by serum withdrawal were unchanged in TSP-1 overexpressing tumor cells. However, TSP-1 overexpression up-regulated the TSP-1 receptor CD36, leading to enhanced adhesion of A431 cells to TSP-1. These findings establish TSP-1 as a potent inhibitor of angiogenesis and tumor growth in carcinomas of the skin.
Figures

References
-
- Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 - PubMed
-
- Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. Exs 1997, 79:233-269 - PubMed
-
- Ferrara N: The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 1995, 36:127-137 - PubMed
-
- Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo K-T, Manseau EJ, Dvorak HF: Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res 1996, 56:172-181 - PubMed
-
- Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE: Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 1997, 3:1222-1227 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

