Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium
- PMID: 10433939
- PMCID: PMC1866847
- DOI: 10.1016/S0002-9440(10)65142-5
Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium
Abstract
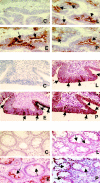
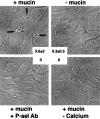
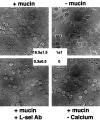
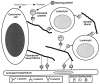
Selectins are adhesion molecules that mediate calcium-dependent cell-cell interactions among leukocytes, platelets, and endothelial cells. The naturally occurring vascular ligands for the selectins are mostly mucin-type glycoproteins. Increased expression and altered glycosylation of mucins are known to be prominent features of carcinoma progression. We have previously shown that all three selectins bind to colon carcinoma cell lines in a calcium-dependent fashion and that carcinoma growth and metastasis formation are attenuated in P-selectin-deficient mice. Here we show that the three recombinant soluble selectins recognize ligands within primary colon carcinoma tissue samples. Affinity chromatography showed that the ligands for all three selectins are O-sialoglycoprotease-sensitive mucins that are recognized in a calcium- and sialic acid-dependent manner. Furthermore, there are separate binding sites on the mucins for each selectin, allowing cross-binding of a single mucin molecule by more than one selectin. We also show that the selectin ligands on purified carcinoma mucins can mediate at least four different pathological interactions among platelets, leukocytes, and endothelial cells. These findings could explain some of the adhesive events of blood-borne tumor cells reported to occur with leukocytes, platelets, and endothelial cells, which are believed to play a part in modulating some early events in tumor metastases.
Figures



References
-
- Rosen SD, Bertozzi CR: The selectins and their ligands. Curr Opin Cell Biol 1994, 6:663-673 - PubMed
-
- Lasky LA: Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem 1995, 64:113-139 - PubMed
-
- Ley K, Tedder TF: Leukocyte interactions with vascular endothelium: New insights into selectin-mediated attachment and rolling. J Immunol 1995, 155:525-528 - PubMed
-
- Nelson RM, Venot A, Bevilacqua MP, Linhardt RJ, Stamenkovic I: Carbohydrate-protein interactions in vascular biology. Annu Rev Cell Biol 1995, 11:601-631 - PubMed
-
- Tedder TF, Steeber DA, Chen A, Engel P: The selectins: vascular adhesion molecules. FASEB J 1995, 9:866-873 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

