Fibrogenesis and fibrolysis in collagenous colitis. Patterns of procollagen types I and IV, matrix-metalloproteinase-1 and -13, and TIMP-1 gene expression
- PMID: 10433942
- PMCID: PMC1866842
- DOI: 10.1016/S0002-9440(10)65145-0
Fibrogenesis and fibrolysis in collagenous colitis. Patterns of procollagen types I and IV, matrix-metalloproteinase-1 and -13, and TIMP-1 gene expression
Abstract
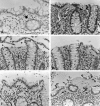
Collagenous colitis is characterized by the deposition of a superficial subepithelial collagenous layer, the pathogenesis of which is unknown. Because the excess matrix deposition is potentially reversible, a labile imbalance between fibrogenesis and fibrolysis may be suspected. Expression of procollagen alpha1(I) and alpha1(IV), matrix-metalloproteinase (MMP)-1 and -13, and tissue inhibitor of metalloproteinase (TIMP)-1 genes was semiquantitated by in situ hybridization on serial biopsies of 12 patients with collagenous colitis and compared to controls. Collagen types I, III, IV, and VI, tenascin, undulin/collagen XIV, and alpha-actin were localized by immunohistology. The superficial collagen layer stained strongly for collagen types I, III, and VI, and particularly for tenascin, but not for undulin. Elevated procollagen alpha1(I), procollagen alpha1(IV), and TIMP-1 transcript levels were found in alpha-actin-positive cells with linear distribution underneath the superficial collagenous layer, whereas MMP-1 RNA expression was variable and restricted to cell clusters. MMP-13 expression was undetectable. The patterns of procollagen alpha1(I)- and alpha1(IV)-specific labeling, combined with an intense tenascin- but absent undulin-specific staining, indicate deposition of an immature interstitial matrix that may be susceptible to degradation. The restricted MMP-1 RNA expression, counteracted by increased TIMP-1 expression, suggests locally impaired fibrolysis as a relevant factor in the pathogenesis of collagenous colitis.
Figures



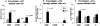

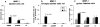
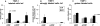
References
-
- Lindström CG: Collagenous colitis with watery diarrhoea: a new entity? Pathol Eur 1976, 11:87-89 - PubMed
-
- Bogomoletz WV: Collagenous, microscopic and lymphocytic colitis: an evolving concept. Virchows Arch 1994, 424:573-579 - PubMed
-
- Bogomoletz WV, Flejou JF: Newly recognized forms of colitis: collagenous colitis, microscopic (lymphocytic) colitis and lymphoid idiopathic proctitis. Semin Diagn Pathol 1991, 8:178-189 - PubMed
-
- Lee E, Schiller LR, Vendrell D, Santa Ana CA, Fordtran JS: Subepithelial collagen table thickness in colon specimens from patients with microscopic colitis and collagenous colitis. Gastroenterology 1992, 103:1780-1796 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

