State-dependent inactivation of the alpha1G T-type calcium channel
- PMID: 10435997
- PMCID: PMC2230639
- DOI: 10.1085/jgp.114.2.185
State-dependent inactivation of the alpha1G T-type calcium channel
Abstract
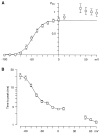
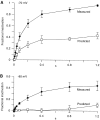
We have examined the kinetics of whole-cell T-current in HEK 293 cells stably expressing the alpha1G channel, with symmetrical Na(+)(i) and Na(+)(o) and 2 mM Ca(2+)(o). After brief strong depolarization to activate the channels (2 ms at +60 mV; holding potential -100 mV), currents relaxed exponentially at all voltages. The time constant of the relaxation was exponentially voltage dependent from -120 to -70 mV (e-fold for 31 mV; tau = 2.5 ms at -100 mV), but tau = 12-17 ms from-40 to +60 mV. This suggests a mixture of voltage-dependent deactivation (dominating at very negative voltages) and nearly voltage-independent inactivation. Inactivation measured by test pulses following that protocol was consistent with open-state inactivation. During depolarizations lasting 100-300 ms, inactivation was strong but incomplete (approximately 98%). Inactivation was also produced by long, weak depolarizations (tau = 220 ms at -80 mV; V(1/2) = -82 mV), which could not be explained by voltage-independent inactivation exclusively from the open state. Recovery from inactivation was exponential and fast (tau = 85 ms at -100 mV), but weakly voltage dependent. Recovery was similar after 60-ms steps to -20 mV or 600-ms steps to -70 mV, suggesting rapid equilibration of open- and closed-state inactivation. There was little current at -100 mV during recovery from inactivation, consistent with </=8% of the channels recovering through the open state. The results are well described by a kinetic model where inactivation is allosterically coupled to the movement of the first three voltage sensors to activate. One consequence of state-dependent inactivation is that alpha1G channels continue to inactivate after repolarization, primarily from the open state, which leads to cumulative inactivation during repetitive pulses.
Figures













References
-
- Aldrich R.W., Stevens C.F. Inactivation of open and closed sodium channels determined separately. Cold Spring Harbor Symp. Quant. Biol. 1983;48:147–153. - PubMed
-
- Armstrong C.M., Matteson D.R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985;227:65–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

