ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin
- PMID: 10436013
- PMCID: PMC25489
- DOI: 10.1091/mbc.10.8.2573
ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin
Abstract
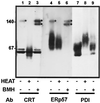
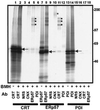
ERp57 is a lumenal protein of the endoplasmic reticulum (ER) and a member of the protein disulfide isomerase (PDI) family. In contrast to archetypal PDI, ERp57 interacts specifically with newly synthesized glycoproteins. In this study we demonstrate that ERp57 forms discrete complexes with the ER lectins, calnexin and calreticulin. Specific ERp57/calreticulin complexes exist in canine pancreatic microsomes, as demonstrated by SDS-PAGE after cross-linking, and by native electrophoresis in the absence of cross-linking. After in vitro translation and import into microsomes, radiolabeled ERp57 can be cross-linked to endogenous calreticulin and calnexin while radiolabeled PDI cannot. Likewise, radiolabeled calreticulin is cross-linked to endogenous ERp57 but not PDI. Similar results were obtained in Lec23 cells, which lack the glucosidase I necessary to produce glycoprotein substrates capable of binding to calnexin and calreticulin. This observation indicates that ERp57 interacts with both of the ER lectins in the absence of their glycoprotein substrate. This result was confirmed by a specific interaction between in vitro synthesized calreticulin and ERp57 prepared in solution in the absence of other ER components. We conclude that ERp57 forms complexes with both calnexin and calreticulin and propose that it is these complexes that can specifically modulate glycoprotein folding within the ER lumen.
Figures



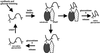
References
-
- Arunachalam B, Cresswell P. Molecular requirements for the interaction of class II major histocompatibility complex molecules and invariant chain with calnexin. J Biol Chem. 1995;270:2784–2790. - PubMed
-
- Baksh S, Burns K, Andrin C, Michalak M. Interaction of calreticulin with protein disulfide isomerase. J Biol Chem. 1995;270:31338–31344. - PubMed
-
- Bourdi M, Demady D, Martin JL, Jabbour SK, Martin BM, George JW, Pohl LR. cDNA cloning and baculovirus expression of the human liver endoplasmic reticulum P58: characterization as a protein disulfide isomerase isoform, but not as a protease or a carnitine acyltransferase. Arch Biochem Biophys. 1995;323:397–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

