Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides
- PMID: 10436021
- PMCID: PMC25498
- DOI: 10.1091/mbc.10.8.2669
Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides
Abstract
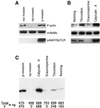
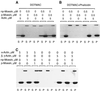
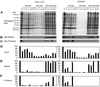
Activation of human platelets with thrombin transiently increases phosphorylation at (558)threonine of moesin as determined with phosphorylation state-specific antibodies. This specific modification is completely inhibited by the kinase inhibitor staurosporine and maximally promoted by the phosphatase inhibitor calyculin A, making it possible to purify the two forms of moesin to homogeneity. Blot overlay assays with F-actin probes labeled with either [32P]ATP or 125I show that only phosphorylated moesin interacts with F-actin in total platelet lysates, in moesin antibody immunoprecipitates, and when purified. In the absence of detergents, both forms of the isolated protein are aggregated. Phosphorylated, purified moesin co-sediments with alpha- or beta/gamma-actin filaments in cationic, but not in anionic, nonionic, or amphoteric detergents. The interaction affinity is high (Kd, approximately 1.5 nM), and the maximal moesin:actin stoichiometry is 1:1. This interaction is also observed in platelets extracted with cationic but not with nonionic detergents. In 0.1% Triton X-100, F-actin interacts with phosphorylated moesin only in the presence of polyphosphatidylinositides. Thus, both polyphosphatidylinositides and phosphorylation can activate moesin's high-affinity F-actin binding site in vitro. Dual regulation by both mechanisms may be important for proper cellular control of moesin-mediated linkages between the actin cytoskeleton and the plasma membrane.
Figures






References
-
- Amieva MR, Furthmayr H. Subcellular localization of moesin in dynamic filopodia, retraction fibers, and other structures involved in substrate exploration, attachment, and cell-cell contacts. Exp Cell Res. 1995;219:180–196. - PubMed
-
- Amieva MR, Litman P, Huang L, Ichimaru E, Furthmayr H. Disruption of dynamic cell surface architecture of NIH3T3 fibroblasts by the N-terminal domains of moesin and ezrin: in vivo imaging with GFP fusion proteins. J Cell Sci. 1998;112:111–125. - PubMed
-
- Benka ML, et al. The thrombin receptor in human platelets is coupled to a GTP binding protein of the G alpha q family. FEBS Lett. 1995;363:49–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

