Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling
- PMID: 10436031
- PMCID: PMC6782848
- DOI: 10.1523/JNEUROSCI.19-16-06740.1999
Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling
Abstract
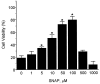
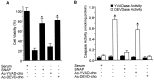
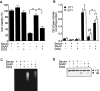
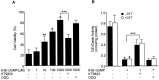
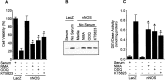
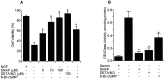
Although nitric oxide (NO) induces neuronal cell death under some conditions, it also can prevent apoptosis resulting from growth factor withdrawal. We investigated the molecular mechanism by which NO protects undifferentiated and differentiated PC12 cells from trophic factor deprivation-induced apoptosis. PC12 cells underwent apoptotic death in association with increased caspase-3-like activity, DNA fragmentation, poly(ADP-ribose) polymerase (PARP) cleavage, and cytochrome c release after 24 hr of serum withdrawal. The apoptosis of PC12 cells was inhibited by the addition of NO-generating donor S-nitroso-N-acetylpenicillamine (SNAP) (5-100 microM) and the specific caspase-3-like protease inhibitor Ac-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-cho) but not the YVADase (or caspase-1-like protease) inhibitor N-acetyl-Tyr-Val-Ala-Asp-aldehyde (Ac-YVAD-cho). SNAP and Ac-DEVD-cho prevented the increase in DEVDase (caspase-3-like protease) activity. The SNAP-mediated suppression of DEVDase activity was only minimally reversed by the incubation of cell lysate with dithiothreitol, indicating that NO did not S-nitrosylate caspase-3-like proteases in PC12 cells. Western blot analysis showed that NO inhibited the proteolytic activation of caspase-3. The cGMP analog 8-bromo-cGMP (8-Br-cGMP) blocked apoptotic cell death, caspase-3 activity and activation, and cytochrome c release. The soluble guanylyl cyclase inhibitor 1-H-oxodiazol-[1,2,4]-[4,3-a] quinoxaline-1-one (CODQ) significantly attenuated NO-mediated, but not 8-Br-cGMP-dependent, inhibition of apoptotic cell death, PARP cleavage, cytochrome c release, and DEVDase activity. Furthermore, the protein kinase G inhibitor KT5823 reversed both SNAP- and 8-Br-cGMP-mediated anti-apoptotic events. All these apoptotic phenomena were also suppressed by NO production through neuronal NO synthase gene transfer into PC12 cells. Furthermore, similar findings were observed in differentiated PC12 cells stimulated to undergo apoptosis by NO donors and NGF deprivation. These findings indicate that NO protects against PC12 cell death by inhibiting the activation of caspase proteases through cGMP production and activation of protein kinase G.
Figures

Similar articles
-
Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation.FASEB J. 2003 Jun;17(9):1036-47. doi: 10.1096/fj.02-0738com. FASEB J. 2003. PMID: 12773486
-
Nitric oxide at a low concentration protects murine macrophage RAW264 cells against nitric oxide-induced death via cGMP signaling pathway.Br J Pharmacol. 2003 May;139(1):28-34. doi: 10.1038/sj.bjp.0705206. Br J Pharmacol. 2003. PMID: 12746220 Free PMC article.
-
Caspase-independent cell death by low concentrations of nitric oxide in PC12 cells: involvement of cytochrome C oxidase inhibition and the production of reactive oxygen species in mitochondria.J Neurosci Res. 2003 Aug 1;73(3):351-63. doi: 10.1002/jnr.10669. J Neurosci Res. 2003. PMID: 12868069
-
Protein thiol modification and apoptotic cell death as cGMP-independent nitric oxide (NO) signaling pathways.Rev Physiol Biochem Pharmacol. 1996;127:1-30. doi: 10.1007/BFb0048263. Rev Physiol Biochem Pharmacol. 1996. PMID: 8533007 Review.
-
The role of 6R-tetrahydrobiopterin in the nervous system.Prog Neurobiol. 2000 Jul;61(4):415-38. doi: 10.1016/s0301-0082(99)00059-3. Prog Neurobiol. 2000. PMID: 10727782 Review.
Cited by
-
Involvement of p38 mitogen-activated protein kinase and apoptosis signal-regulating kinase-1 in nitric oxide-induced cell death in PC12 cells.Neurochem Res. 2001 May;26(5):525-32. doi: 10.1023/a:1010917129951. Neurochem Res. 2001. PMID: 11513480
-
Guanylyl Cyclase A/cGMP Signaling Slows Hidden, Age- and Acoustic Trauma-Induced Hearing Loss.Front Aging Neurosci. 2020 Apr 9;12:83. doi: 10.3389/fnagi.2020.00083. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32327991 Free PMC article.
-
Treatment with Mesna and n-3 polyunsaturated fatty acids ameliorates experimental ulcerative colitis in rats.Int J Exp Pathol. 2015 Dec;96(6):433-43. doi: 10.1111/iep.12163. Epub 2016 Jan 19. Int J Exp Pathol. 2015. PMID: 26852691 Free PMC article.
-
cGMP-Phosphodiesterase Inhibition Prevents Hypoxia-Induced Cell Death Activation in Porcine Retinal Explants.PLoS One. 2016 Nov 18;11(11):e0166717. doi: 10.1371/journal.pone.0166717. eCollection 2016. PLoS One. 2016. PMID: 27861632 Free PMC article.
-
Nitric oxide and promotion of cardiac myocyte apoptosis.Mol Cell Biochem. 2004 Aug;263(1):35-53. doi: 10.1023/B:MCBI.0000041847.63338.b8. Mol Cell Biochem. 2004. PMID: 27520664
References
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
-
- Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993;7:349–360. - PubMed
-
- Arnelle DR, Stamler JS. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;18:279–285. - PubMed
-
- Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP. Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of β-amyloid precursor. J Neurochem. 1995;64:2087–2096. - PubMed
-
- Beauvais F, Michel L, Dubertret L. The nitric oxide donors, azide and hydroxylamine, inhibit the programmed cell death of cytokine-deprived human eosinophils. FEBS Lett. 1995;361:229–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
