Active role of glutamate uptake in the synaptic transmission from retinal nonspiking neurons
- PMID: 10436033
- PMCID: PMC6782855
- DOI: 10.1523/JNEUROSCI.19-16-06755.1999
Active role of glutamate uptake in the synaptic transmission from retinal nonspiking neurons
Abstract
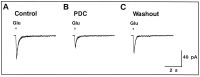
We examined the role of glutamate uptake in the synaptic transmission of graded responses from newt retinal bipolar cells (BCs) to ganglion layer cells (GLCs). In dissociated Müller cells (retinal glia), glutamate evoked an uptake current that was effectively inhibited by L-trans-pyrrolidine-2,4-dicarboxylic acid (PDC). PDC had no effect on the non-NMDA receptors of dissociated spiking neurons. In the retinal slice preparation, dual whole-cell recordings were performed from a pair of BC and GLC. A depolarizing pulse applied to a BC activated the Ca(2+) current (I(Ca)) in the BC and evoked an EPSC in the GLC. Application of PDC prolonged both non-NMDA and NMDA receptor-mediated components of the evoked EPSC but changed neither the amplitude nor time course of I(Ca). When the slice preparation was superfused with a solution containing glutamate but not PDC, the evoked EPSC decreased in amplitude without changing the time course, suggesting that the prolongation of the evoked EPSC is not attributable to a simple increase in ambient glutamate concentration after inhibition of glutamate uptake. Because PDC did not affect the amplitude, time course, or frequency of spontaneous EPSCs, it is unlikely that PDC modified presynaptic and/or postsynaptic mechanisms. These results indicate that inhibition of glutamate uptake slows the clearance of glutamate accumulated in the synaptic cleft by multiple quantal release and prolongs the evoked EPSC. The role of glutamate uptake at synapses in the inner retina is not only to maintain the extracellular glutamate concentration at a low level but also to terminate the light-evoked EPSCs rapidly.
Figures








References
-
- Ball AK, Dickson DH. Displaced amacrine and ganglion cells in the newt retina. Exp Eye Res. 1983;36:199–213. - PubMed
-
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. - PubMed
-
- Bridges RJ, Stanley MS, Anderson MW, Cotman CW, Chamberlin AR. Conformationally defined neurotransmitter analogues. Selective inhibition of glutamate uptake by one pyrrolidine-2:4-dicarboxylate diastereomer. J Med Chem. 1991;34:717–725. - PubMed
-
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
