Identification of residues in the N terminus of alpha1B critical for inhibition of the voltage-dependent calcium channel by Gbeta gamma
- PMID: 10436043
- PMCID: PMC6782846
- DOI: 10.1523/JNEUROSCI.19-16-06855.1999
Identification of residues in the N terminus of alpha1B critical for inhibition of the voltage-dependent calcium channel by Gbeta gamma
Abstract
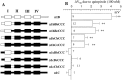
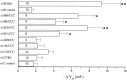
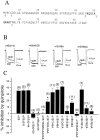
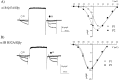
To examine the role of the intracellular N terminus in the G-protein modulation of the neuronal voltage-dependent calcium channel (VDCC) alpha1B, we have pursued two routes of investigation. First, we made chimeric channels between alpha1B and alpha1C, the latter not being modulated by Gbeta gamma subunits. VDCC alpha1 subunit constructs were coexpressed with accessory alpha2delta and beta2a subunits in Xenopus oocytes and mammalian (COS-7) cells. G-protein modulation of expressed alpha1 subunits was induced by activation of coexpressed dopamine (D2) receptors with quinpirole in oocytes, or by cotransfection of Gbeta1gamma2 subunits in COS-7 cells. For the chimeric channels, only those with the N terminus of alpha1B showed any G-protein modulation; further addition of the first transmembrane domain and I-II intracellular linker of alpha1B increased the degree of modulation. To determine the amino acids within the alpha1B N terminus, essential for G-protein modulation, we made mutations of this sequence and identified three amino acids (S48, R52, and R54) within an 11 amino acid sequence as being critical for G-protein modulation, with I49 being involved to a lesser extent. This sequence may comprise an essential part of a complex Gbeta gamma-binding site or be involved in its subsequent action.
Figures




Similar articles
-
Identification of the amino terminus of neuronal Ca2+ channel alpha1 subunits alpha1B and alpha1E as an essential determinant of G-protein modulation.J Neurosci. 1998 Jul 1;18(13):4815-24. doi: 10.1523/JNEUROSCI.18-13-04815.1998. J Neurosci. 1998. PMID: 9634547 Free PMC article.
-
Interaction between G proteins and accessory subunits in the regulation of 1B calcium channels in Xenopus oocytes.J Physiol. 2000 Sep 15;527 Pt 3(Pt 3):419-32. doi: 10.1111/j.1469-7793.2000.t01-1-00419.x. J Physiol. 2000. PMID: 10990530 Free PMC article.
-
Role of domain I of neuronal Ca2+ channel alpha1 subunits in G protein modulation.J Physiol. 1998 May 15;509 ( Pt 1)(Pt 1):163-9. doi: 10.1111/j.1469-7793.1998.163bo.x. J Physiol. 1998. PMID: 9547390 Free PMC article.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
Structures and functions of calcium channel beta subunits.J Bioenerg Biomembr. 1998 Aug;30(4):357-75. doi: 10.1023/a:1021989622656. J Bioenerg Biomembr. 1998. PMID: 9758332 Review.
Cited by
-
G-protein inhibition of N- and P/Q-type calcium channels: distinctive elementary mechanisms and their functional impact.J Neurosci. 2001 Feb 15;21(4):1137-47. doi: 10.1523/JNEUROSCI.21-04-01137.2001. J Neurosci. 2001. PMID: 11160384 Free PMC article.
-
The alpha1B Ca2+ channel amino terminus contributes determinants for beta subunit-mediated voltage-dependent inactivation properties.J Physiol. 2000 Jun 1;525 Pt 2(Pt 2):377-90. doi: 10.1111/j.1469-7793.2000.t01-1-00377.x. J Physiol. 2000. PMID: 10835041 Free PMC article.
-
The stargazin-related protein gamma 7 interacts with the mRNA-binding protein heterogeneous nuclear ribonucleoprotein A2 and regulates the stability of specific mRNAs, including CaV2.2.J Neurosci. 2008 Oct 15;28(42):10604-17. doi: 10.1523/JNEUROSCI.2709-08.2008. J Neurosci. 2008. PMID: 18923037 Free PMC article.
-
Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology.J Physiol. 2016 Oct 1;594(19):5369-90. doi: 10.1113/JP272262. Epub 2016 Jul 5. J Physiol. 2016. PMID: 27273705 Free PMC article. Review.
-
N terminus is key to the dominant negative suppression of Ca(V)2 calcium channels: implications for episodic ataxia type 2.J Biol Chem. 2010 Jan 8;285(2):835-44. doi: 10.1074/jbc.M109.065045. Epub 2009 Nov 10. J Biol Chem. 2010. PMID: 19903821 Free PMC article.
References
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage-dependence. Nature. 1989;340:153–155. - PubMed
-
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarisation-sensitive α1A antibody. Eur J Neurosci. 1997;9:749–759. - PubMed
-
- Campbell V, Berrow N, Brickley K, Page K, Wade R, Dolphin AC. Voltage-dependent calcium channel β-subunits in combination with alpha1 subunits have a GTPase activating effect to promote hydrolysis of GTP by Gαo in rat frontal cortex. FEBS Lett. 1995a;370:135–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
