Thyroid hormone regulates reelin and dab1 expression during brain development
- PMID: 10436054
- PMCID: PMC6782883
- DOI: 10.1523/JNEUROSCI.19-16-06979.1999
Thyroid hormone regulates reelin and dab1 expression during brain development
Abstract
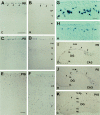
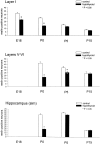
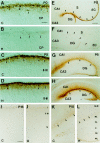
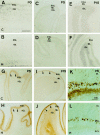
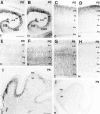
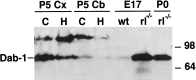
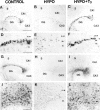
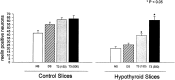
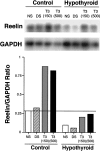
The reelin and dab1 genes are necessary for appropriate neuronal migration and lamination during brain development. Since these processes are controlled by thyroid hormone, we studied the effect of thyroid hormone deprivation and administration on the expression of reelin and dab1. As shown by Northern analysis, in situ hybridization, and immunohistochemistry studies, hypothyroid rats expressed decreased levels of reelin RNA and protein during the perinatal period [embryonic day 18 (E18) and postnatal day 0 (P0)]. The effect was evident in Cajal-Retzius cells of cortex layer I, as well as in layers V/VI, hippocampus, and granular neurons of the cerebellum. At later ages, however, Reelin was more abundant in the cortex, hippocampus, cerebellum, and olfactory bulb of hypothyroid rats (P5), and no differences were detected at P15. Conversely, Dab1 levels were higher at P0, and lower at P5 in hypothyroid animals. In line with these results, reelin RNA and protein levels were higher in cultured hippocampal slices from P0 control rats compared to those from hypothyroid animals. Significantly, thyroid-dependent regulation of reelin and dab1 was confirmed in vivo and in vitro by hormone treatment of hypothyroid rats and organotypic cultures, respectively. In both cases, thyroid hormone led to an increase in reelin expression. Our data suggest that the effects of thyroid hormone on neuronal migration may be in part mediated through the control of reelin and dab1 expression during brain ontogenesis.
Figures

References
-
- Alvarez-Dolado M, Iglesias T, Rodríguez-Peña A, Bernal J, Muñoz A. Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Mol Brain Res. 1994;27:249–257. - PubMed
-
- Alvarez-Dolado M, González-Sancho J, Bernal J, Muñoz A. Developmental expression of tenascin-C is altered by hypothyroidism in the rat brain. Neuroscience. 1998;84:309–322. - PubMed
-
- Berbel P, Guadaño-Ferraz A, Martínez M, Quiles J, Balboa R, Innocenti G. Organization of auditory callosal connections in hypothyroid rats. Eur J Neurosci. 1993;5:1465–1478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
