Nitric oxide in the retinotectal system: a signal but not a retrograde messenger during map refinement and segregation
- PMID: 10436061
- PMCID: PMC6782861
- DOI: 10.1523/JNEUROSCI.19-16-07066.1999
Nitric oxide in the retinotectal system: a signal but not a retrograde messenger during map refinement and segregation
Abstract
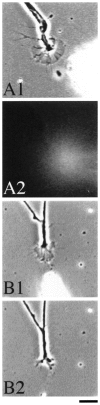
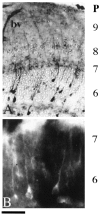
The role of nitric oxide (NO) as a mediator of synaptic plasticity is controversial in both the adult and developing brain. NO generation appears to be necessary for some types of NMDA receptor-dependent synaptic plasticity during development but not for others. Our previous work using several NO donors revealed that Xenopus laevis retinal ganglion cell axons stop growing in response to NO exposure. We demonstrate here that the same response occurs in tectal neuron processes bathed in the NO donor S-nitrosocysteine (SNOC) and in RGC growth cones to which SNOC is very locally applied. We show that NO synthase (NOS) activity is present in the Rana pipiens optic tectum throughout development in a dispersed subpopulation of tectal neurons, although effects of NO on synaptic function in a Rana pipiens tectal slice were varied. We chronically inhibited NOS in doubly innervated Rana tadpole optic tecta using L-N(G)-nitroarginine methyl ester in Elvax. Despite significant NOS inhibition as measured biochemically, eye-specific stripes remained normally segregated. This suggests that NOS activity is not downstream of NMDA receptor activation during retinotectal synaptic competition because NMDA receptor activation is necessary for segregation of retinal afferents into ocular dominance stripes in the doubly innervated tadpole optic tectum. We conclude that NO has some signaling function in the retinotectal pathway, but this function is not critical to the mechanism that refines the projection and causes eye-specific stripes.
Figures



Similar articles
-
NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection.J Neurosci. 1990 Apr;10(4):1197-216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. J Neurosci. 1990. PMID: 2158526 Free PMC article.
-
Mechanisms involved in development of retinotectal connections: roles of Eph receptor tyrosine kinases, NMDA receptors and nitric oxide.Prog Brain Res. 1998;118:115-31. doi: 10.1016/s0079-6123(08)63204-5. Prog Brain Res. 1998. PMID: 9932438 Review.
-
Nicotine exposure refines visual map topography through an NMDA receptor-mediated pathway.Eur J Neurosci. 2006 Dec;24(11):3026-42. doi: 10.1111/j.1460-9568.2006.05204.x. Eur J Neurosci. 2006. PMID: 17156364
-
NMDA receptor-mediated refinement of a transient retinotectal projection during development requires nitric oxide.J Neurosci. 1999 Jan 1;19(1):229-35. doi: 10.1523/JNEUROSCI.19-01-00229.1999. J Neurosci. 1999. PMID: 9870953 Free PMC article.
-
The contribution of protein kinases to plastic events in the superior colliculus.Prog Neuropsychopharmacol Biol Psychiatry. 1997 Apr;21(3):487-505. doi: 10.1016/s0278-5846(97)00014-6. Prog Neuropsychopharmacol Biol Psychiatry. 1997. PMID: 9153069 Review.
Cited by
-
Nitric oxide is an essential negative regulator of cell proliferation in Xenopus brain.J Neurosci. 2001 Nov 15;21(22):8809-18. doi: 10.1523/JNEUROSCI.21-22-08809.2001. J Neurosci. 2001. PMID: 11698593 Free PMC article.
-
Co-induction of growth-associated protein GAP-43 and neuronal nitric oxide synthase in the cochlear nucleus following cochleotomy.Exp Brain Res. 2004 Sep;158(2):151-62. doi: 10.1007/s00221-004-1886-1. Epub 2004 May 18. Exp Brain Res. 2004. PMID: 15148562
-
The potential role of nitric oxide synthase in survival and regeneration of magnocellular neurons of hypothalamo-neurohypophyseal system.Neurochem Res. 2009 Nov;34(11):1907-13. doi: 10.1007/s11064-009-9965-0. Epub 2009 Apr 21. Neurochem Res. 2009. PMID: 19381805
-
A competition-based mechanism mediates developmental refinement of tectal neuron receptive fields.J Neurosci. 2012 Nov 21;32(47):16872-9. doi: 10.1523/JNEUROSCI.2372-12.2012. J Neurosci. 2012. PMID: 23175839 Free PMC article.
-
The role of nitric oxide in development of topographic precision in the retinotectal projection of chick.J Neurosci. 2001 Jun 15;21(12):4318-25. doi: 10.1523/JNEUROSCI.21-12-04318.2001. J Neurosci. 2001. PMID: 11404417 Free PMC article.
References
-
- Ankri N, Legendre P, Faber DS, Korn H. Automatic detection of spontaneous synaptic responses in central neurons. J Neurosci Methods. 1994;52:87–100. - PubMed
-
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J Comp Neurol. 1996;369:64–82. - PubMed
-
- Blanton MG, LoTurco JJ, Kriegstein AR. Whole cell recordings from neurons in slices of reptilian and mammalian cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
-
- Bruning G, Mayer B. Localization of nitric oxide synthase in the brain of the frog, Xenopus laevis. Brain Res. 1996;741:331–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
