Motion opponency in visual cortex
- PMID: 10436069
- PMCID: PMC6782843
- DOI: 10.1523/JNEUROSCI.19-16-07162.1999
Motion opponency in visual cortex
Abstract
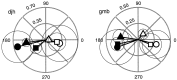
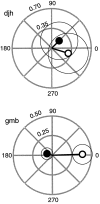
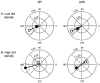
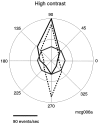
Perceptual studies suggest that visual motion perception is mediated by opponent mechanisms that correspond to mutually suppressive populations of neurons sensitive to motions in opposite directions. We tested for a neuronal correlate of motion opponency using functional magnetic resonance imaging (fMRI) to measure brain activity in human visual cortex. There was strong motion opponency in a secondary visual cortical area known as the human MT complex (MT+), but there was little evidence of motion opponency in primary visual cortex. To determine whether the level of opponency in human and monkey are comparable, a variant of these experiments was performed using multiunit electrophysiological recording in areas MT and MST of the macaque monkey brain. Although there was substantial variability in the degree of opponency between recording sites, the monkey and human data were qualitatively similar on average. These results provide further evidence that: (1) direction-selective signals underly human MT+ responses, (2) neuronal signals in human MT+ support visual motion perception, (3) human MT+ is homologous to macaque monkey MT and adjacent motion sensitive brain areas, and (4) that fMRI measurements are correlated with average spiking activity.
Figures





Similar articles
-
Motion opponency and transparency in the human middle temporal area.Eur J Neurosci. 2009 Sep;30(6):1172-82. doi: 10.1111/j.1460-9568.2009.06893.x. Epub 2009 Sep 1. Eur J Neurosci. 2009. PMID: 19723288
-
Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey.J Neurosci. 1994 Jul;14(7):4109-24. doi: 10.1523/JNEUROSCI.14-07-04109.1994. J Neurosci. 1994. PMID: 8027765 Free PMC article.
-
Perceptual learning of motion direction discrimination with suppressed and unsuppressed MT in humans: an fMRI study.PLoS One. 2013;8(1):e53458. doi: 10.1371/journal.pone.0053458. Epub 2013 Jan 9. PLoS One. 2013. PMID: 23326433 Free PMC article.
-
Linking Neuronal Direction Selectivity to Perceptual Decisions About Visual Motion.Annu Rev Vis Sci. 2020 Sep 15;6:335-362. doi: 10.1146/annurev-vision-121219-081816. Annu Rev Vis Sci. 2020. PMID: 32936737 Review.
-
Delineating extrastriate visual area MT(V5) using cortical myeloarchitecture.Neuroimage. 2014 Jun;93 Pt 2:231-6. doi: 10.1016/j.neuroimage.2013.03.034. Epub 2013 Mar 26. Neuroimage. 2014. PMID: 23541801 Review.
Cited by
-
Orientation selectivity of motion-boundary responses in human visual cortex.J Neurophysiol. 2010 Dec;104(6):2940-50. doi: 10.1152/jn.00400.2010. Epub 2010 Sep 22. J Neurophysiol. 2010. PMID: 20861432 Free PMC article.
-
Motion processing, directional selectivity, and conscious visual perception in the human brain.Proc Natl Acad Sci U S A. 2008 Oct 21;105(42):16362-7. doi: 10.1073/pnas.0802867105. Epub 2008 Oct 8. Proc Natl Acad Sci U S A. 2008. PMID: 18843114 Free PMC article.
-
Shifts in the population response in the middle temporal visual area parallel perceptual and motor illusions produced by apparent motion.J Neurosci. 2001 Dec 1;21(23):9387-402. doi: 10.1523/JNEUROSCI.21-23-09387.2001. J Neurosci. 2001. PMID: 11717372 Free PMC article. Clinical Trial.
-
Low-level mechanisms do not explain paradoxical motion percepts.J Vis. 2010 Apr 28;10(4):20.1-9. doi: 10.1167/10.4.20. J Vis. 2010. PMID: 20465339 Free PMC article.
-
Single-Trial fMRI Decoding of 3D Motion with Stereoscopic and Perspective Cues.J Neurosci. 2025 May 28;45(22):e0044252025. doi: 10.1523/JNEUROSCI.0044-25.2025. J Neurosci. 2025. PMID: 40262902 Free PMC article.
References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am. 1985;2:284–299. - PubMed
-
- Albrecht DG, Geisler WS. Motion sensitivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci. 1991;7:531–546. - PubMed
-
- Albright TD. Cortical processing of visual motion. In: Miles FA, Wallman J, editors. Visual motion and its role in the stabilization of gaze. Elsevier; Amsterdam: 1993. pp. 177–201. - PubMed
-
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol. 1997;78:516–520. - PubMed
-
- Beckers G, Homberg V. Cerebral visual motion blindness: Transitory akinetopsia induced by transcranial magnetic stimulation of human area V5. Proc R Soc Lond B Biol Sci. 1992;249:173–178. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
