Inhibition of antigen presentation by the glycine/alanine repeat domain is not conserved in simian homologues of Epstein-Barr virus nuclear antigen 1
- PMID: 10438828
- PMCID: PMC104265
- DOI: 10.1128/JVI.73.9.7381-7389.1999
Inhibition of antigen presentation by the glycine/alanine repeat domain is not conserved in simian homologues of Epstein-Barr virus nuclear antigen 1
Abstract
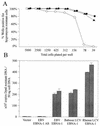
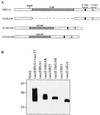
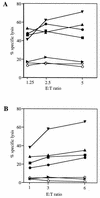
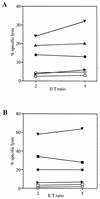
Most humans and Old World nonhuman primates are infected for life with Epstein-Barr virus (EBV) or closely related gammaherpesviruses in the same lymphocryptovirus (LCV) subgroup. Several potential strategies for immune evasion and persistence have been proposed based on studies of EBV infection in humans, but it has been difficult to test their actual contribution experimentally. Interest has focused on the EBV nuclear antigen 1 (EBNA1) because of its essential role in the maintenance and replication of the episomal viral genome in latently infected cells and because EBNA1 endogenously expressed in these cells is protected from presentation to the major histocompatibility complex class-I restricted cytotoxic T-lymphocyte (CTL) response through the action of an internal glycine-alanine repeat (GAR). Given the high degree of biologic conservation among LCVs which infect humans and Old World primates, we hypothesized that strategies essential for viral persistence would be well conserved among viruses of this subgroup. We show that the rhesus LCV EBNA1 shares sequence homology with the EBV and baboon LCV EBNA1 and that the rhesus LCV EBNA1 is a functional homologue for EBV EBNA1-dependent plasmid maintenance and replication. Interestingly, all three LCVs possess a GAR domain, but the baboon and rhesus LCV EBNA1 GARs fail to inhibit antigen processing and presentation as determined by using three different in vitro CTL assays. These studies suggest that inhibition of antigen processing and presentation by the EBNA1 GAR may not be an essential mechanism for persistent infection by all LCV and that other mechanisms may be important for immune evasion during LCV infection.
Figures


References
-
- Allen T M, Sidney J, del Guercio M F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol. 1998;160:6062–6071. - PubMed
-
- Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla M G, Frappier L, Rickinson A. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. - PubMed
-
- Blasco R, Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157–162. - PubMed
-
- Falk K, Gratama J W, Rowe M, Zou J Z, Khanim F, Young L S, Oosterveer M A, Ernberg I. The role of repetitive DNA sequences in the size variation of Epstein-Barr virus (EBV) nuclear antigens, and the identification of different EBV isolates using RFLP and PCR analysis. J Gen Virol. 1995;76:779–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

