Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251
- PMID: 10438833
- PMCID: PMC104270
- DOI: 10.1128/JVI.73.9.7430-7440.1999
Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251
Abstract
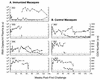
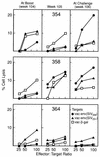
Rhesus macaques were immunized with a combination vaccine regimen consisting of adenovirus type 5 host range mutant-simian immunodeficiency virus envelope (Ad5hr-SIVenv) recombinant priming and boosting with native SIV gp120. Upon intravaginal challenge with SIVmac251, both persistently and transiently viremic animals were observed (S. L. Buge, E. Richardson, S. Alipanah, P. Markham, S. Cheng, N. Kalyan, C. J. Miller, M. Lubeck, S. Udem, J. Eldridge, and M. Robert-Guroff, J. Virol. 71:8531-8541, 1997). Long-term follow-up of the persistently viremic immunized macaques, which displayed significantly reduced viral burdens during the first 18 weeks postchallenge compared to controls, has now shown that one of four became a slow progressor, clearing virus from plasma and remaining asymptomatic with stable CD4 counts for 134 weeks postchallenge. Reboosting of the transiently viremic macaques did not reactivate latent virus. Rechallenge with two sequential SIVmac251 intravaginal exposures again resulted in partial protection of one of two immunized macaques, manifested by viral clearance and stable CD4 counts. No single immune parameter was associated with partial protection. Development of a strong antibody response capable of neutralizing a primary SIVmac251 isolate together with SIV-specific cytotoxic T lymphocytes were implicated, while CD8(+) T-cell antiviral activity and mucosal immune responses were not associated with delayed disease progression. Our data show that even a third immunization with the same Ad5hr-SIVenv recombinant can elicit significant immune responses to the inserted gene product, suggesting that preexisting Ad antibodies may not preclude effective immunization. Further, the partial protection against a virulent, pathogenic SIV challenge observed in two of six macaques immunized with a vaccine regimen based solely on the viral envelope indicates that this vectored-vaccine approach has promise and that multicomponent vaccines based in the same system merit further investigation.
Figures







References
-
- Alexander N J. Sexual transmission of human immunodeficiency virus: virus entry into the male and female genital tract. Fertil Steril. 1990;54:1–18. - PubMed
-
- Almond H, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. - PubMed
-
- Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
-
- Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Pennick D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. - PubMed
-
- Barker E, Bossart K N, Fujimura S H, Levy J A. CD28 costimulation increases CD8+ cell suppression of HIV replication. J Immunol. 1997;159:5123–5131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

