Induction of apoptosis in murine coronavirus-infected cultured cells and demonstration of E protein as an apoptosis inducer
- PMID: 10438879
- PMCID: PMC104316
- DOI: 10.1128/JVI.73.9.7853-7859.1999
Induction of apoptosis in murine coronavirus-infected cultured cells and demonstration of E protein as an apoptosis inducer
Abstract
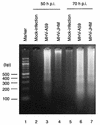
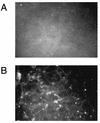
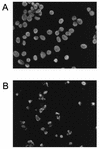
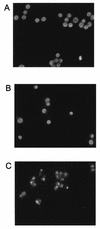
We demonstrated that infection of 17Cl-1 cells with the murine coronavirus mouse hepatitis virus (MHV) induced caspase-dependent apoptosis. MHV-infected DBT cells did not show apoptotic changes, indicating that apoptosis was not a universal mechanism of cell death in MHV-infected cells. Expression of MHV structural proteins by recombinant vaccinia viruses showed that expression of MHV E protein induced apoptosis in DBT cells, whereas expression of other MHV structural proteins, including S protein, M protein, N protein, and hemagglutinin-esterase protein, failed to induce apoptosis. MHV E protein-mediated apoptosis was suppressed by a high level of Bcl-2 oncogene expression. Our data showed that MHV E protein is a multifunctional protein; in addition to its known function in coronavirus envelope formation, it also induces apoptosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

