Localization in the nucleolus and coiled bodies of protein subunits of the ribonucleoprotein ribonuclease P
- PMID: 10444065
- PMCID: PMC2150555
- DOI: 10.1083/jcb.146.3.559
Localization in the nucleolus and coiled bodies of protein subunits of the ribonucleoprotein ribonuclease P
Abstract
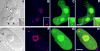
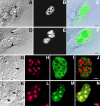
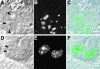
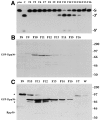
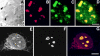
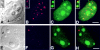
The precise location of the tRNA processing ribonucleoprotein ribonuclease P (RNase P) and the mechanism of its intranuclear distribution have not been completely delineated. We show that three protein subunits of human RNase P (Rpp), Rpp14, Rpp29 and Rpp38, are found in the nucleolus and that each can localize a reporter protein to nucleoli of cells in tissue culture. In contrast to Rpp38, which is uniformly distributed in nucleoli, Rpp14 and Rpp29 are confined to the dense fibrillar component. Rpp29 and Rpp38 possess functional, yet distinct domains required for subnucleolar localization. The subunit Rpp14 lacks such a domain and appears to be dependent on a piggyback process to reach the nucleolus. Biochemical analysis suggests that catalytically active RNase P exists in the nucleolus. We also provide evidence that Rpp29 and Rpp38 reside in coiled bodies, organelles that are implicated in the biogenesis of several other small nuclear ribonucleoproteins required for processing of precursor mRNA. Because some protein subunits of RNase P are shared by the ribosomal RNA processing ribonucleoprotein RNase MRP, these two evolutionary related holoenzymes may share common intranuclear localization and assembly pathways to coordinate the processing of tRNA and rRNA precursors.
Figures



References
-
- Antoine M., Reimers K., Dickson C., Kiefer P. Fibroblast growth factor 3, a protein with dual subcellular localization, is targeted to the nucleus and nucleolus by the concerted action of two nuclear localization signals and a nucleolar retention signal. J. Biol. Chem. 1997;272:29475–29481. - PubMed
-
- Bachant J.B., Elledge S.J. Mitotic treasures in the nucleolus. Nature. 1999;398:757–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

