Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila
- PMID: 10444068
- PMCID: PMC2150560
- DOI: 10.1083/jcb.146.3.597
Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila
Abstract
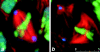
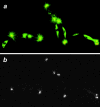
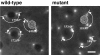
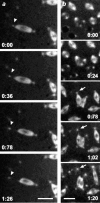
Cytoplasmic dynein is a multisubunit minus-end-directed microtubule motor that serves multiple cellular functions. Genetic studies in Drosophila and mouse have demonstrated that dynein function is essential in metazoan organisms. However, whether the essential function of dynein reflects a mitotic requirement, and what specific mitotic tasks require dynein remains controversial. Drosophila is an excellent genetic system in which to analyze dynein function in mitosis, providing excellent cytology in embryonic and somatic cells. We have used previously characterized recessive lethal mutations in the dynein heavy chain gene, Dhc64C, to reveal the contributions of the dynein motor to mitotic centrosome behavior in the syncytial embryo. Embryos lacking wild-type cytoplasmic dynein heavy chain were analyzed by in vivo analysis of rhodamine-labeled microtubules, as well as by immunofluorescence in situ methods. Comparisons between wild-type and Dhc64C mutant embryos reveal that dynein function is required for the attachment and migration of centrosomes along the nuclear envelope during interphase/prophase, and to maintain the attachment of centrosomes to mitotic spindle poles. The disruption of these centrosome attachments in mutant embryos reveals a critical role for dynein function and centrosome positioning in the spatial organization of the syncytial cytoplasm of the developing embryo.
Figures






References
-
- Allan V. Organelle movement. Dynactinportrait of a dynein regulator. Curr. Biol. 1994;4:1000–1002. - PubMed
-
- Boleti H., Karsenti E., Vernos I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. - PubMed
-
- Busson S., Dujardin D., Moreau A., Dompierre J., De Mey J.R. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;8:541–544. - PubMed
-
- Callaini G., Dallai R., Riparbelli M.G. Cytochalasin induces spindle fusion in the syncytial blastoderm of the early Drosophila embryo. Biol. Cell. 1992;74:249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

