Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4
- PMID: 10444069
- PMCID: PMC2150549
- DOI: 10.1083/jcb.146.3.609
Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4
Abstract
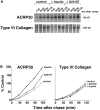
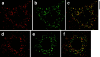
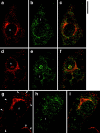
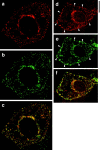
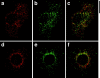
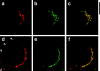
Insulin stimulates adipose cells both to secrete proteins and to translocate the GLUT4 glucose transporter from an intracellular compartment to the plasma membrane. We demonstrate that whereas insulin stimulation of 3T3-L1 adipocytes has no effect on secretion of the alpha3 chain of type VI collagen, secretion of the protein hormone adipocyte complement related protein of 30 kD (ACRP30) is markedly enhanced. Like GLUT4, regulated exocytosis of ACRP30 appears to require phosphatidylinositol-3-kinase activity, since insulin-stimulated ACRP30 secretion is blocked by pharmacologic inhibitors of this enzyme. Thus, 3T3-L1 adipocytes possess a regulated secretory compartment containing ACRP30. Whether GLUT4 recycles to such a compartment has been controversial. We present deconvolution immunofluorescence microscopy data demonstrating that the subcellular distributions of ACRP30 and GLUT4 are distinct and nonoverlapping; in contrast, those of GLUT4 and the transferrin receptor overlap. Together with supporting evidence that GLUT4 does not recycle to a secretory compartment via the trans-Golgi network, we conclude that there are at least two compartments that undergo insulin-stimulated exocytosis in 3T3-L1 adipocytes: one for ACRP30 secretion and one for GLUT4 translocation.
Figures


References
-
- Balla T., Downing G.J., Jaffe H., Kim S., Zolyomi A., Catt K.J. Isolation and molecular cloning of wortmannin-sensitive bovine type III phosphatidylinositol 4-kinases. J. Biol. Chem. 1997;272:18358–18366. - PubMed
-
- Barr V.A., Malide D., Zarnowski M., Taylor S.I., Cushman S.W. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–4472. - PubMed
-
- Benito M., Porras A., Nebreda A., Santos E. Differentiation of 3T3-L1 fibroblasts to adipocytes induced by transfection of ras oncogenes. Science. 1991;253:565–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

