I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure
- PMID: 10444071
- PMCID: PMC2150553
- DOI: 10.1083/jcb.146.3.631
I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure
Abstract
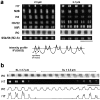
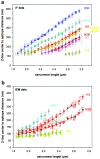
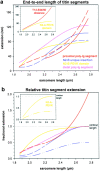
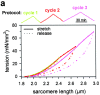
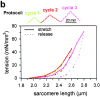
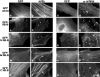
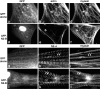
In cardiac muscle, the giant protein titin exists in different length isoforms expressed in the molecule's I-band region. Both isoforms, termed N2-A and N2-B, comprise stretches of Ig-like modules separated by the PEVK domain. Central I-band titin also contains isoform-specific Ig-motifs and nonmodular sequences, notably a longer insertion in N2-B. We investigated the elastic behavior of the I-band isoforms by using single-myofibril mechanics, immunofluorescence microscopy, and immunoelectron microscopy of rabbit cardiac sarcomeres stained with sequence-assigned antibodies. Moreover, we overexpressed constructs from the N2-B region in chick cardiac cells to search for possible structural properties of this cardiac-specific segment. We found that cardiac titin contains three distinct elastic elements: poly-Ig regions, the PEVK domain, and the N2-B sequence insertion, which extends approximately 60 nm at high physiological stretch. Recruitment of all three elements allows cardiac titin to extend fully reversibly at physiological sarcomere lengths, without the need to unfold Ig domains. Overexpressing the entire N2-B region or its NH(2) terminus in cardiac myocytes greatly disrupted thin filament, but not thick filament structure. Our results strongly suggest that the NH(2)-terminal N2-B domains are necessary to stabilize thin filament integrity. N2-B-titin emerges as a unique region critical for both reversible extensibility and structural maintenance of cardiac myofibrils.
Figures



References
-
- Allen D.G., Kentish J.C. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 1985;17:821–840. - PubMed
-
- Broschat K.O. Tropomyosin prevents depolymerization of actin filaments from the pointed end. J. Biol. Chem. 1990;265:21323–21329. - PubMed
-
- Erickson H.P. Stretching single protein moleculestitin is a weird spring. Science. 1997;276:1090–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

