Hydrophobic protein synthesized in the pod endocarp adheres to the seed surface
- PMID: 10444078
- PMCID: PMC59354
- DOI: 10.1104/pp.120.4.951
Hydrophobic protein synthesized in the pod endocarp adheres to the seed surface
Abstract
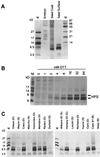
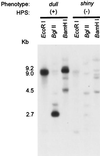
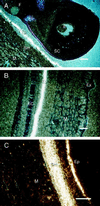
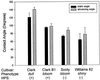
Soybean (Glycine max [L.] Merr.) hydrophobic protein (HPS) is an abundant seed constituent and a potentially hazardous allergen that causes asthma in persons allergic to soybean dust. By analyzing surface extracts of soybean seeds with sodium dodecyl sulfate-polyacrylamide gel electrophoresis and amino-terminal microsequencing, we determined that large amounts of HPS are deposited on the seed surface. The quantity of HPS present varies among soybean cultivars and is more prevalent on dull-seeded phenotypes. We have also isolated cDNA clones encoding HPS and determined that the preprotein is translated with a membrane-spanning signal sequence and a short hydrophilic domain. Southern analysis indicated that multiple copies of the HPS gene are present in the soybean genome, and that the HPS gene structure is polymorphic among cultivars that differ in seed coat luster. The pattern of HPS gene expression, determined by in situ hybridization and RNA analysis, shows that HPS is synthesized in the endocarp of the inner ovary wall and is deposited on the seed surface during development. This study demonstrates that a seed dust allergen is associated with the seed luster phenotype in soybean and that compositional properties of the seed surface may be altered by manipulating gene expression in the ovary wall.
Figures



References
-
- Antó JM, Sunyer J, Reed CE, Sabrià J, Martínez F, Morrell F, Cordina R, Rodriguez-Roisin R, Rodrigo MJ, Roco J. Preventing asthma epidemics due to soybeans by dust-control measures. N Engl J Med. 1993;329:1760–1763. - PubMed
-
- Antó JM, Sunyer J, Rodriguez-Roisin R, Suarez-Cervera M, Vazquez L. Community outbreaks of asthma associated with inhalation of soybean dust. N Engl J Med. 1989;320:1097–1102. - PubMed
-
- Baud F, Pebay-Peyroula E, Cohen-Addad C, Odani S, Lehmann MS. Crystal structure of hydrophobic protein from soybean: a member of a new cysteine-rich family. J Mol Biol. 1993;231:877–887. - PubMed
-
- Bernard RL, Weiss MG (1973) Qualitative genetics. In BE Caldwell, ed, Soybeans: Improvement, Production, and Uses. American Society of Agronomy, Madison, WI, pp 117–154
-
- Blum H, Beier H, Gross HJ. Improved staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

