Fyn and Lck tyrosine kinases regulate tyrosine phosphorylation of p105CasL, a member of the p130Cas docking protein family, in T-cell receptor-mediated signalling
- PMID: 10447714
- PMCID: PMC2326814
- DOI: 10.1046/j.1365-2567.1999.00753.x
Fyn and Lck tyrosine kinases regulate tyrosine phosphorylation of p105CasL, a member of the p130Cas docking protein family, in T-cell receptor-mediated signalling
Abstract
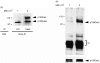
We have previously shown that engagement of the T-cell receptor (TCR)/CD3 complex with anti-CD3 antibody induces tyrosine phosphorylation of p105CasL (CasL), a member of the p130Cas docking protein family. In the present work, we attempted to determine which protein tyrosine kinases (PTKs) regulate TCR-mediated phosphorylation of CasL. We show here that an association between CasL and two types of Src family PTKs, Fyn and Lck, is induced by anti-CD3 cross-linking of human H9 T cells. In contrast, ZAP-70, another PTK that also plays a critical role in the TCR signalling, failed to bind CasL, even after anti-CD3 stimulation. In vitro kinase assays revealed that Fyn and Lck, but not ZAP-70, were capable of phosphorylating CasL. Moreover, we found that CasL was constitutively hyperphosphorylated in vivo in splenocytes of MRL-MP-lpr/lpr mice, in which overproduction and excessive activation of Fyn and Lck have previously been shown to occur. Constitutive in vivo binding of CasL to both kinases was also demonstrated in lpr splenocytes. These results strongly suggest that CasL is a substrate for Fyn and Lck PTKs in TCR signal transduction.
Figures



Similar articles
-
Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk.Eur J Immunol. 1997 Aug;27(8):2113-7. doi: 10.1002/eji.1830270840. Eur J Immunol. 1997. PMID: 9295052
-
Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn.Mol Cell Biol. 2000 Feb;20(4):1426-35. doi: 10.1128/MCB.20.4.1426-1435.2000. Mol Cell Biol. 2000. PMID: 10648627 Free PMC article.
-
Interaction between Sam68 and Src family tyrosine kinases, Fyn and Lck, in T cell receptor signaling.J Biol Chem. 1997 Mar 7;272(10):6214-9. doi: 10.1074/jbc.272.10.6214. J Biol Chem. 1997. PMID: 9045636
-
T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases.Cell. 1993 Apr 23;73(2):209-12. doi: 10.1016/0092-8674(93)90221-b. Cell. 1993. PMID: 8477442 Review. No abstract available.
-
The role of tyrosine kinases and phosphotyrosine-containing recognition motifs in regulation of the T cell-antigen receptor-mediated signal transduction pathway.J Leukoc Biol. 1994 Feb;55(2):265-71. doi: 10.1002/jlb.55.2.265. J Leukoc Biol. 1994. PMID: 7507972 Review.
Cited by
-
Tyrosine kinases EnAbling adaptor molecules for chemokine-induced Rap1 activation in T cells.Sci Signal. 2012 Jul 31;5(235):pe33. doi: 10.1126/scisignal.2003383. Sci Signal. 2012. PMID: 22855504 Free PMC article.
-
Abl family kinases modulate T cell-mediated inflammation and chemokine-induced migration through the adaptor HEF1 and the GTPase Rap1.Sci Signal. 2012 Jul 17;5(233):ra51. doi: 10.1126/scisignal.2002632. Sci Signal. 2012. PMID: 22810897 Free PMC article.
-
De novo generation of dual-target compounds using artificial intelligence.iScience. 2024 Dec 17;28(1):111526. doi: 10.1016/j.isci.2024.111526. eCollection 2025 Jan 17. iScience. 2024. PMID: 39801837 Free PMC article.
-
Profiling of tyrosine phosphorylation pathways in human cells using mass spectrometry.Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):443-8. doi: 10.1073/pnas.2436191100. Epub 2003 Jan 9. Proc Natl Acad Sci U S A. 2003. PMID: 12522270 Free PMC article.
-
CAS proteins in normal and pathological cell growth control.Cell Mol Life Sci. 2010 Apr;67(7):1025-48. doi: 10.1007/s00018-009-0213-1. Epub 2009 Nov 25. Cell Mol Life Sci. 2010. PMID: 19937461 Free PMC article. Review.
References
-
- Ishino M, Ohba T, Sasaki H, Sasaki T. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene. 1995;11:2331. - PubMed
-
- Alexandropoulos K, Baltimore D. Coordinateactivation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

