Importance of thioredoxin in the proteolysis of an immunoglobulin G as antigen by lysosomal Cys-proteases
- PMID: 10447715
- PMCID: PMC2326805
- DOI: 10.1046/j.1365-2567.1999.00748.x
Importance of thioredoxin in the proteolysis of an immunoglobulin G as antigen by lysosomal Cys-proteases
Abstract
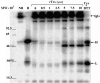
For disulphide-bonded antigens, reduction has been postulated to be a prerequisite for proteolytic antigen processing, with subsequent production of major histocompatibility complex (MHC) class II binding fragments. The murine monoclonal immunoglobulin G (IgG) CE25/B7 was used as a multimeric antigen in a human model. Native IgG is highly resistant to proteolysis and has been previously found to be partially reduced at early steps of cell processing to become a suitable substrate for endopeptidases. The role of the oxidoreductase thioredoxin (Trx) was assessed in the reduction of the IgG by cleavage of H-L and H-H disulphide bonds. Recombinant human Trx (rTrx) has been assayed in a proteolytic in vitro system on IgG using endosomal and lysosomal subcellular fractions from B lymphoblastoid cells. rTrx is required in a dose-dependent manner for development of efficient proteolysis, catalysed by thiol-dependent Cys-proteases, such as cathepsin B. We demonstrated that cathepsin B activity was stimulated by the addition of rTrx. Thus, we propose that Trx-dependent IgG proteolysis occurred, on the one hand by means of the unfolding of the IgG after disulphide reduction, becoming a substrate of lysosomal proteases, and on the other hand by Cys-proteases such as cathepsin B that are fully active upon the regeneration of their activity by hydrogen donors.
Figures

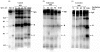



References
-
- Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Ann Rev Cell Dev Biol. 1995;11:267. - PubMed
-
- Hampl J, Gradehandt G, Plachov D, et al. Presentation of insulin and insulin A chain peptides to mouse T cells: involvement of cysteine residues. Mol Immunol. 1991;28:479. - PubMed
-
- Jensen PE. Acidification and disulfide reduction can be sufficient to allow intact proteins to bind class II MHC. J Immunol. 1993;150:3347. - PubMed
-
- Merkel BJ, Mandel R, Ryser HJ, McCoy KL. Characterization of fibroblasts with a unique defect in processing antigens with disulfide bonds. J Immunol. 1995;154:128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

