Interleukin-15 production at the early stage after oral infection with Listeria monocytogenes in mice
- PMID: 10447719
- PMCID: PMC2326806
- DOI: 10.1046/j.1365-2567.1999.00752.x
Interleukin-15 production at the early stage after oral infection with Listeria monocytogenes in mice
Abstract
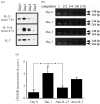
We previously reported that exogenous interleukin-15 (IL-15) induces proliferation and activation of intestinal intraepithelial lymphocytes (i-IEL) in naive mice. To investigate the ability of endogenous IL-15 to stimulate i-IEL in vivo, we monitored i-IEL and intestinal epithelial cells (i-EC) in mice after an oral infection with Listeria monocytogenes. Although the populations of alphabeta and gammadelta i-IEL were not significantly changed after the oral infection, the expression level of interferon-gamma (IFN-gamma) was increased both at transcriptional and protein levels, and a conversely marked decrease in interleukin-4 (IL-4) was detected in the i-IEL on day 1 after infection as compared with before infection. The T helper 1 (Th1)-biased response of i-IEL coincided with a peak response of IL-15 production in the i-EC after oral infection. These results suggested that IL-15 produced from i-EC may be at least partly involved in the stimulation of i-IEL to produce IFN-gamma after oral infection with L. monocytogenes.
Figures


Similar articles
-
Interleukin-15 may be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats.Infect Immun. 1998 Dec;66(12):5677-83. doi: 10.1128/IAI.66.12.5677-5683.1998. Infect Immun. 1998. PMID: 9826341 Free PMC article.
-
The interaction of intestinal epithelial cells and intraepithelial lymphocytes in host defense.Immunol Res. 1999;20(3):219-35. doi: 10.1007/BF02790405. Immunol Res. 1999. PMID: 10741862 Review.
-
Influence of beta 2-microglobulin expression on gamma interferon secretion and target cell lysis by intraepithelial lymphocytes during intestinal Listeria monocytogenes infection.Infect Immun. 1996 Feb;64(2):569-75. doi: 10.1128/iai.64.2.569-575.1996. Infect Immun. 1996. PMID: 8550209 Free PMC article.
-
Listeria monocytogenes-induced gamma interferon secretion by intestinal intraepithelial gamma/delta T lymphocytes.Infect Immun. 1993 May;61(5):2154-61. doi: 10.1128/iai.61.5.2154-2161.1993. Infect Immun. 1993. PMID: 8478105 Free PMC article.
-
Interleukin-4 and listeriosis.Immunol Rev. 1997 Aug;158:95-105. doi: 10.1111/j.1600-065x.1997.tb00995.x. Immunol Rev. 1997. PMID: 9314077 Review.
Cited by
-
CD8 alpha-deficient mice are highly susceptible to 5-fluorouracil-induced lethality.Clin Diagn Lab Immunol. 2002 May;9(3):550-7. doi: 10.1128/cdli.9.3.550-557.2002. Clin Diagn Lab Immunol. 2002. PMID: 11986258 Free PMC article.
-
Early gene response of human brain microvascular endothelial cells to Listeria monocytogenes infection.Can J Microbiol. 2011 May;57(5):441-6. doi: 10.1139/w11-018. Epub 2011 May 5. Can J Microbiol. 2011. PMID: 21542783 Free PMC article.
-
Irradiation with a low-level diode laser induces the developmental endothelial locus-1 gene and reduces proinflammatory cytokines in epithelial cells.Lasers Med Sci. 2014 May;29(3):987-94. doi: 10.1007/s10103-013-1439-6. Epub 2013 Oct 3. Lasers Med Sci. 2014. PMID: 24197516
-
Soluble interleukin-15 complexes are generated in vivo by type I interferon dependent and independent pathways.PLoS One. 2015 Mar 10;10(3):e0120274. doi: 10.1371/journal.pone.0120274. eCollection 2015. PLoS One. 2015. PMID: 25756182 Free PMC article.
-
Interleukin-15 enhances cytotoxicity, receptor expression, and expansion of neonatal natural killer cells in long-term culture.Clin Diagn Lab Immunol. 2004 Sep;11(5):879-88. doi: 10.1128/CDLI.11.5.879-888.2004. Clin Diagn Lab Immunol. 2004. PMID: 15358647 Free PMC article.
References
-
- Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965. - PubMed
-
- Tagaya Y, Bamford RN, Defilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

