Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1
- PMID: 10447720
- PMCID: PMC2326819
- DOI: 10.1046/j.1365-2567.1999.00746.x
Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1
Abstract
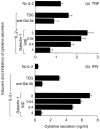
The migration of immune cells through the extracellular matrix (ECM) towards inflammatory sites is co-ordinated by receptors recognizing ECM glycoproteins, chemokines and proinflammatory cytokines. In this context, galectins are secreted to the extracellular milieu, where they recognize poly-N-acetyllactosamine chains on major ECM glycoproteins, such as fibronectin and laminin. We investigated the possibility that galectin-1 could modulate the adhesion of human T cells to ECM and ECM components. T cells were purified from human blood, activated with interleukin-2 (IL-2), labelled, and incubated further with intact immobilized ECM and ECM glycoproteins in the presence of increasing concentrations of human recombinant galectin-1, or its more stable, related, C2-S molecule obtained by site-directed mutagenesis. The presence of galectin-1 was shown to inhibit T-cell adhesion to intact ECM, laminin and fibronectin, and to a lesser extent to collagen type IV, in a dose-dependent manner. This effect was specifically blocked by anti-galectin-1 antibody and was dependent on the lectin's carbohydrate-binding properties. The inhibition of T-cell adhesion by galectin-1 correlates with the ability of this molecule to block the re-organization of the activated cell's actin cytoskeleton. Furthermore, tumour necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma) production was markedly reduced when IL-2-activated T cells were incubated with galectin-1 or its mutant. This effect was prevented by beta-galactoside-related sugars. The present study reveals an alternative inhibitory mechanism for explaining the suppressive properties of the galectin-1 subfamily on inflammatory and autoimmune processes.
Figures

References
-
- Barondes SH, Cooper DNW, Gitt MA, Leffler H. Galectins: structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807. - PubMed
-
- Barondes SH, Castronovo V, Cooper DNW, et al. Galectin: a family of animal β-galactoside-binding lectins. Cell. 1994;76:597. - PubMed
-
- Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem. 1996;119:1. - PubMed
-
- Hirabayashi J, Kasai K. The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297. - PubMed
-
- Poirrier F, Robertson EJ. Normal development of mice carrying a null mutation in the gene encoding the L-14 S-type lectin. Development. 1993;119:1229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

