Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection
- PMID: 10447721
- PMCID: PMC2326813
- DOI: 10.1046/j.1365-2567.1999.00723.x
Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection
Abstract
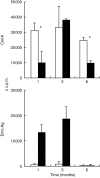
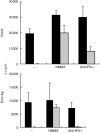
In some parasitic infections immunosuppression is a prominent characteristic of the host-parasite interplay. We have used a murine alveolar echinococcosis (AE) model in susceptible C57BL/6 mice to document a suppressed splenocyte proliferative response to concanavalin A (Con A) at the early (1-month) stage and to Echinococcus multilocularis-crude antigen (Emc-antigen) at the late (4-6-month) stage of chronic infection. Despite proliferative suppression, splenic cytokine production [interleukin-2 (IL-2), IL-4 and interferon-gamma (IFN-gamma)] in response to Con A or Emc-antigen stimulation was not suppressed at 1 month postinfection (p.i.). Infection resulted in a strong Mac-1+ cell infiltration of the peritoneal cavity and spleen. Peritoneal cells (PEC) from mice infected at the 1-month stage were rich in macrophages and expressed significantly higher levels of transcripts for the inflammatory cytokine IL-1beta and for tumour necrosis factor-alpha and inducible nitric oxide synthase (iNOS), when compared with PEC from non-infected control mice. Conversely, the IL-10 transcript level remained low and did not change during infection. Spleen cells supplemented with PEC from infected mice induced a marked increase in the levels of nitrite in response to Con A and Emc-antigen stimulation, and also a complete suppression of splenic proliferation. The spleen cells from late-stage infected mice expressed only background levels of IL-10 but greatly increased levels of iNOS, when compared with normal spleen cells. This observation correlated with the immunosuppression demonstrated at the late stage of murine AE. Furthermore, the suppressed splenic proliferative responses observed at the early and late stage were reversed to a large extent by the addition of NG-monomethyl-l-arginine and partially by anti-IFN-gamma. Thus, our results demonstrated that the immunosuppression observed in chronic AE was not primarily dependent on IL-10 but rather on nitric oxide production by macrophages from infected animals.
Figures



Similar articles
-
Inducible nitric oxide synthase deficiency in mice increases resistance to chronic infection with Echinococcus multilocularis.Immunology. 2003 Feb;108(2):238-44. doi: 10.1046/j.1365-2567.2003.01567.x. Immunology. 2003. PMID: 12562333 Free PMC article.
-
Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative response to concanavalin A and toxoplasma antigen during acute toxoplasmosis.Infect Immun. 1995 Mar;63(3):751-6. doi: 10.1128/iai.63.3.751-756.1995. Infect Immun. 1995. PMID: 7868243 Free PMC article.
-
Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria.J Leukoc Biol. 1995 Jul;58(1):23-31. doi: 10.1002/jlb.58.1.23. J Leukoc Biol. 1995. PMID: 7542305
-
Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection.J Immunol. 1995 Oct 15;155(8):3955-63. J Immunol. 1995. PMID: 7561103
-
Molecular survival strategies of Echinococcus multilocularis in the murine host.Parasitol Int. 2006;55 Suppl:S45-9. doi: 10.1016/j.parint.2005.11.006. Epub 2005 Dec 13. Parasitol Int. 2006. PMID: 16352460 Review.
Cited by
-
Cellular and humoral peritoneal immunity to Mesocestoides vogae metacestode infection in mice.Parasit Vectors. 2021 Jan 18;14(1):54. doi: 10.1186/s13071-020-04541-0. Parasit Vectors. 2021. PMID: 33461599 Free PMC article.
-
A DNA vaccine encoding for TcSSP4 induces protection against acute and chronic infection in experimental Chagas disease.Int J Biol Sci. 2011;7(9):1230-8. doi: 10.7150/ijbs.7.1230. Epub 2011 Oct 25. Int J Biol Sci. 2011. PMID: 22110377 Free PMC article.
-
Pathological Lesions and Inducible Nitric Oxide Synthase Expressions in the Liver of Mice Experimentally Infected with Clonorchis sinensis.Korean J Parasitol. 2015 Dec;53(6):777-83. doi: 10.3347/kjp.2015.53.6.777. Epub 2015 Dec 31. Korean J Parasitol. 2015. PMID: 26797449 Free PMC article.
-
Concepts in immunology and diagnosis of hydatid disease.Clin Microbiol Rev. 2003 Jan;16(1):18-36. doi: 10.1128/CMR.16.1.18-36.2003. Clin Microbiol Rev. 2003. PMID: 12525423 Free PMC article. Review.
-
Increase of Vascular Endothelial Growth Factor and Decrease of MCP-1 and Some Updated Epidemiology Aspects of Cystic Echinococcosis Human Cases in Calabria Region.Mediators Inflamm. 2018 Jan 14;2018:4283672. doi: 10.1155/2018/4283672. eCollection 2018. Mediators Inflamm. 2018. PMID: 29535593 Free PMC article.
References
-
- Gottstein B, Hemphill A. Immunopathology of echinococcosis. Chem Immunol. 1997;66:177. - PubMed
-
- Gregory SH, Wing EJ, Hoffman RA, Simmons RL. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993;150:2901. - PubMed
-
- Bresson-Hadni S, Liance M, Meyer JP, Houin R, Bresson JL, Vuitton DA. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol. 1990;82:378. - PMC - PubMed
-
- Liance M, Vuitton DA, Guerret-Stocker S, Carbillet JP, Grimaud JA, Houin R. Experimental alveolar echinococcosis. Suitability of a murine model of intrahepatic infection by Echinococcus multilocularis for immunological studies. Experientia. 1984;40:1436. - PubMed
-
- Playford MC, Ooi HK, Oku Y, Kamiya M. Secondary Echinococcus multilocularis infection in severe combined immunodeficient (scid) mice: biphasic growth of the larval cyst mass. Int J Parasitol. 1992;22:975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

