VCAM-1 contributes to rapid eosinophil accumulation induced by the chemoattractants PAF and LTB4: evidence for basal expression of functional VCAM-1 in rat skin
- PMID: 10447726
- PMCID: PMC2326804
- DOI: 10.1046/j.1365-2567.1999.00766.x
VCAM-1 contributes to rapid eosinophil accumulation induced by the chemoattractants PAF and LTB4: evidence for basal expression of functional VCAM-1 in rat skin
Abstract
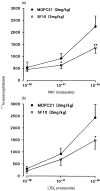
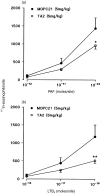
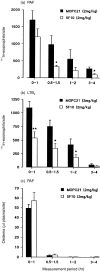
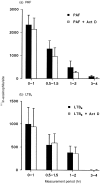
The aim of the present study was to investigate the role of the adhesion pathway alpha4 integrins/vascular cell adhesion molecule type 1 (VCAM-1) in rapid eosinophil accumulation induced by the chemoattractants PAF and LTB4. For this purpose we have used an in vivo model of local 111In-eosinophil accumulation to quantify eosinophil accumulation induced by intradermal administration of platelet-activating factor (PAF) and leukotriene B4 (LTB4) in rats. Initial experiments carried out over 4 hr demonstrated that intravenous administration of an anti-VCAM-1 monoclonal antibody (mAb; 5F10) or an anti-alpha4 integrin mAb (TA2) caused a significant reduction in PAF- or LTB4-induced 111In-labelled eosinophil accumulation. Time-course experiments demonstrated that the anti-VCAM-1 mAb was effective at suppressing early phases of the 111In-labelled eosinophil accumulation induced by PAF and LTB4 (e.g. within the first 60 min). In contrast, 111In-labelled eosinophil accumulation induced by these chemoattractants was unaffected by the local administration of the transcriptional inhibitor actinomycin D, suggesting a role for basally expressed VCAM-1. Indeed, basal expression of VCAM-1 in rat skin sites was demonstrated by the localization of intravenously administered radiolabelled mAb. The localization of the radiolabelled antibody was not altered in skin sites injected with PAF or LTB4. Finally, the inhibitory effects seen with the anti-VCAM-1 mAb were enhanced when the antibody was co-injected into rats with an anti-intercellular adhesion molecule-1 (ICAM-1) mAb (1A29). The combination of these two mAb also caused a significant inhibition of PAF-induced oedema, as quantified by the local accumulation of 125I-labelled human serum albumin. The results indicate a role for alpha4 integrins/VCAM-1 and ICAM-1, in PAF- and LTB4-induced eosinophil accumulation in vivo and suggest that basally expressed VCAM-1 may have a functional role in rapid accumulation of eosinophils induced by chemoattractants.
Figures
Similar articles
-
Tumor necrosis factor alpha-induced eosinophil accumulation in rat skin is dependent on alpha4 integrin/vascular cell adhesion molecule-1 adhesion pathways.Blood. 1997 Nov 15;90(10):4144-52. Blood. 1997. PMID: 9354685
-
IL-1 is a potent inducer of eosinophil accumulation in rat skin. Inhibition of response by a platelet-activating factor antagonist and an anti-human IL-8 antibody.J Immunol. 1995 Feb 1;154(3):1364-73. J Immunol. 1995. PMID: 7822803
-
IL-4-induced eosinophil accumulation in rat skin is dependent on endogenous TNF-alpha and alpha 4 integrin/VCAM-1 adhesion pathways.J Immunol. 1998 Jun 1;160(11):5637-45. J Immunol. 1998. PMID: 9605170
-
Human eotaxin induces alpha 4 and beta 2 integrin-dependent eosinophil accumulation in rat skin in vivo: delayed generation of eotaxin in response to IL-4.J Immunol. 1998 Apr 1;160(7):3569-76. J Immunol. 1998. PMID: 9531320
-
Adhesion molecules in cutaneous immunity.Semin Immunopathol. 2007 Apr;29(1):45-57. doi: 10.1007/s00281-007-0065-4. Semin Immunopathol. 2007. PMID: 17621953 Review.
Cited by
-
Diagnosis and management of eosinophilic asthma: a US perspective.J Asthma Allergy. 2014 Apr 11;7:53-65. doi: 10.2147/JAA.S39119. eCollection 2014. J Asthma Allergy. 2014. PMID: 24748808 Free PMC article. Review.
-
alpha(4) integrin-dependent eosinophil recruitment in allergic but not non-allergic inflammation.Br J Pharmacol. 2001 Jan;132(2):596-604. doi: 10.1038/sj.bjp.0703857. Br J Pharmacol. 2001. PMID: 11159710 Free PMC article.
-
Inhibiting Th1/2 cells influences hepatic capillarization by adjusting sinusoidal endothelial fenestrae through Rho-ROCK-myosin pathway.Aging (Albany NY). 2021 Feb 1;13(4):5069-5086. doi: 10.18632/aging.202425. Epub 2021 Feb 1. Aging (Albany NY). 2021. PMID: 33535174 Free PMC article.
-
Leukotriene B4 mediates gammadelta T lymphocyte migration in response to diverse stimuli.J Leukoc Biol. 2010 Feb;87(2):323-32. doi: 10.1189/jlb.0809563. Epub 2009 Oct 30. J Leukoc Biol. 2010. PMID: 19880577 Free PMC article.
References
-
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068. - PubMed
-
- Sanz M, Hartnell A, Chisholm P, et al. Tumor necrosis factor α-induced eosinophil accumulation in rat skin is dependent on α4 integrin/vascular cell adhesion molecule-1 adhesion pathways. Blood. 1997;90:4144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

