FcepsilonRI-mediated antigen endocytosis turns interferon-gamma-treated mouse mast cells from inefficient into potent antigen-presenting cells
- PMID: 10447750
- PMCID: PMC2326822
- DOI: 10.1046/j.1365-2567.1999.00789.x
FcepsilonRI-mediated antigen endocytosis turns interferon-gamma-treated mouse mast cells from inefficient into potent antigen-presenting cells
Abstract
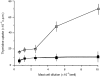
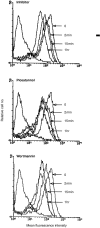
Previous studies in our laboratory have shown that bone-marrow-derived mast cells (BMMC) could present immunogenic peptides, from soluble antigens endocytosed through fluid phase, only if they were subjected to a 48-hr treatment with interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF). In contrast to GM-CSF, interferon-gamma (IFN-gamma) which highly upregulates major histocompatibility complex (MHC) class II expression, completely inhibits the generation of immunogenic peptides. We have used this model to study the role of FcepsilonRI-mediated antigen internalization in the regulation of the antigen-presenting function of IFN-gamma-treated mast cells. Here, we report that FcepsilonRI can reverse the IFN-gamma-treated mast cells from inefficient to highly efficient antigen-presenting cells. Inhibition of the antigen presenting capacity by piceatannol, a protein tyrosine kinase (PTK) syk inhibitor, indicates that this is an active process resulting from immunoglobulin E (IgE)-antigen-FcepsilonRI engagement which involves tyrosines found in the immunoreceptor tyrosine-based activation motif (ITAM) embedded in the cytoplasmic tail of the FcepsilonRI beta and gamma chains. Antigen-presenting function was also shown to require the activation of phosphatidyl inositol 3 (PI3) kinase, downstream of PTK syk phosphorylation, since this activity was completely blocked by wortmannin, a PI3 kinase inhibitor. These data suggest that signalling generated by FcepsilonRI provides mast cells with IgE-mediated enhanced antigen presentation to T cells and emphasize a so far unknown immunoregulatory mast-cell function that might take place in inflammatory sites.
Figures
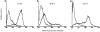


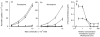
Similar articles
-
The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II.BMC Immunol. 2010 Jun 30;11:34. doi: 10.1186/1471-2172-11-34. BMC Immunol. 2010. PMID: 20591187 Free PMC article.
-
Presentation of soluble antigens by mast cells: upregulation by interleukin-4 and granulocyte/macrophage colony-stimulating factor and downregulation by interferon-gamma.Cell Immunol. 1995 Jun;163(1):37-46. doi: 10.1006/cimm.1995.1096. Cell Immunol. 1995. PMID: 7758129
-
Exogenous and endogenous antigens are differentially presented by mast cells to CD4+ T lymphocytes.Eur J Immunol. 1996 Oct;26(10):2517-28. doi: 10.1002/eji.1830261036. Eur J Immunol. 1996. PMID: 8898968
-
Unexpected functions of FcepsilonRI on antigen-presenting cells.Int Arch Allergy Immunol. 2001 Jan-Mar;124(1-3):35-7. doi: 10.1159/000053662. Int Arch Allergy Immunol. 2001. PMID: 11306920 Review.
-
The role of the FcepsilonRI beta-chain in allergic diseases.Int Arch Allergy Immunol. 2004 Sep;135(1):62-72. doi: 10.1159/000080231. Epub 2004 Aug 13. Int Arch Allergy Immunol. 2004. PMID: 15316148 Review.
Cited by
-
Capacity of mouse mast cells to prime T cells and to induce specific antibody responses in vivo.Immunology. 2001 Feb;102(2):165-72. doi: 10.1046/j.1365-2567.2001.01178.x. Immunology. 2001. PMID: 11260321 Free PMC article.
-
Akkermansia muciniphila exacerbates food allergy in fibre-deprived mice.Nat Microbiol. 2023 Oct;8(10):1863-1879. doi: 10.1038/s41564-023-01464-1. Epub 2023 Sep 11. Nat Microbiol. 2023. PMID: 37696941 Free PMC article.
-
Indirect involvement of allergen-captured mast cells in antigen presentation.Blood. 2008 Feb 1;111(3):1489-96. doi: 10.1182/blood-2007-07-102111. Epub 2007 Nov 21. Blood. 2008. PMID: 18032707 Free PMC article.
-
Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell?Nat Rev Immunol. 2014 Nov;14(11):719-30. doi: 10.1038/nri3754. Epub 2014 Oct 17. Nat Rev Immunol. 2014. PMID: 25324123 Review.
-
Fc receptors and their role in immune regulation and autoimmunity.J Clin Immunol. 2005 Jan;25(1):1-18. doi: 10.1007/s10875-005-0353-8. J Clin Immunol. 2005. PMID: 15742153 Review.
References
-
- Pirron U, Schlunck T, Prinz JC, Rieber EP. IgE-dependent antigen focusing by human B lymphocytes is mediated by the low-affinity receptor for IgE. Eur J Immunol. 1990;20:1547. - PubMed
-
- Squire MC, Studer EJ, Lees A, Finkelman FD, Conrad DH. Antigen presentation is enhanced by targeting antigen to FcεRII by antigen-anti-FcεRII conjugates. J Immunol. 1994;152:4388. - PubMed
-
- Heyman B, Tianmin L, Gustavsson S. In vivo enhancement of the specific antibody response via the low-affinity receptor for IgE. Eur J Immunol. 1993;23:1739. - PubMed
-
- Gustavsson S, Hjulstrom S, Liu T, Heyman B. CD23/IgE-mediated regulation of the specific antibody response in vivo. J Immunol. 1994;152:4793. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

