Influence of the mu-chain C-terminal sequence on polymerization of immunoglobulin M
- PMID: 10447761
- PMCID: PMC2326861
- DOI: 10.1046/j.1365-2567.1999.00797.x
Influence of the mu-chain C-terminal sequence on polymerization of immunoglobulin M
Abstract
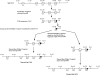
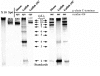
Immunoglobulin (IgM) is found in various states of covalent polymerization (microL)n, where n is typically 8, 10, or 12. The usual form of IgM of bony fish is tetrameric (8 microL units) as compared to the pentameric form (10 microL units) observed in cartilaginous fish and mammals. Two hypotheses were tested in this study. First, that the length of the mu-chain C terminus following Cys575 determines whether an IgM polymerizes as a tetramer or as a pentamer. This was tested by examining the covalent polymerization state of mouse IgM mutated to contain a series of mu-chain C-termini from bony and cartilaginous fish. The results proved this hypothesis wrong: mouse IgM bearing the C-terminal sequence of shark, salmon and cod mu-chain behaved identically to native mouse IgM, forming predominantly (microL)10 and (microL)12 forms. The second hypothesis was that an additional Cys residue near the C terminus of the mu-chain is responsible for the multiple covalent structures seen in IgM of the channel catfish. The addition of a catfish C terminus to the mouse mu-chain resulted, as predicted, in the production of a series of covalently bonded forms, with the major species being (microL)4. When a Ser-Cys unit was removed from the catfish C terminus added to the mouse mu-chain, this resulted in production of IgM indistinguishable in structure from that of wild-type mouse IgM.
Figures


Similar articles
-
Cloning and sequence analysis of channel catfish heavy chain cDNA indicate phylogenetic diversity within the IgM immunoglobulin family.J Immunol. 1989 Feb 15;142(4):1356-65. J Immunol. 1989. PMID: 2492581
-
Analysis of IgM structures involved in J chain incorporation.J Immunol. 1997 Feb 15;158(4):1719-26. J Immunol. 1997. PMID: 9029108
-
Expression of a mouse-channel catfish chimeric IgM molecule in a mouse myeloma cell.Mol Immunol. 1993 Nov;30(16):1405-17. doi: 10.1016/0161-5890(93)90102-h. Mol Immunol. 1993. PMID: 8232326
-
Varied redox forms of teleost IgM: an alternative to isotypic diversity?Immunol Rev. 1998 Dec;166:133-42. doi: 10.1111/j.1600-065x.1998.tb01258.x. Immunol Rev. 1998. PMID: 9914908 Review.
-
Evolutionary variation of immunoglobulin mu heavy chain RNA processing pathways: origins, effects, and implications.Immunol Rev. 1998 Dec;166:143-51. doi: 10.1111/j.1600-065x.1998.tb01259.x. Immunol Rev. 1998. PMID: 9914909 Review.
Cited by
-
Immunoglobulin isotypes in Atlantic salmon, Salmo salar.Biomolecules. 2015 Feb 27;5(1):166-77. doi: 10.3390/biom5010166. Biomolecules. 2015. PMID: 25734583 Free PMC article. Review.
-
Structure of the catfish IGH locus: analysis of the region including the single functional IGHM gene.Immunogenetics. 2006 Oct;58(10):831-44. doi: 10.1007/s00251-006-0139-9. Epub 2006 Aug 29. Immunogenetics. 2006. PMID: 16941126
-
Stellabody: A novel hexamer-promoting mutation for improved IgG potency.Immunol Rev. 2024 Nov;328(1):438-455. doi: 10.1111/imr.13400. Epub 2024 Oct 4. Immunol Rev. 2024. PMID: 39364646 Free PMC article. Review.
-
Artificial surface labelling of Escherichia coli with StrepTagII antigen to study how monoclonal antibodies drive complement-mediated killing.Sci Rep. 2023 Nov 1;13(1):18836. doi: 10.1038/s41598-023-46026-x. Sci Rep. 2023. PMID: 37914798 Free PMC article.
-
Structure, Function, and Therapeutic Use of IgM Antibodies.Antibodies (Basel). 2020 Oct 13;9(4):53. doi: 10.3390/antib9040053. Antibodies (Basel). 2020. PMID: 33066119 Free PMC article. Review.
References
-
- Brewer JW, Randall TD, Parkhouse RME, Corley RB. Mechanism and subcellular localization of secretory IgM polymer assembly. J Biol Chem. 1994;269:17338. - PubMed
-
- Pascual D, Clem LW. Ligand binding by murine IgM antibodies: Intramolecular heterogeneity exists in certain, but not all, cases. Mol Immunol. 1988;25:87. - PubMed
-
- Davis AC, Roux KH, Shulman MJ. On the structure of polymeric IgM. Eur J Immunol. 1988;18:1001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

