Target-specific control of nicotinic receptor expression at developing interneuronal synapses in chick
- PMID: 10448217
- PMCID: PMC2280032
- DOI: 10.1038/9183
Target-specific control of nicotinic receptor expression at developing interneuronal synapses in chick
Abstract
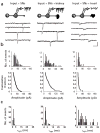
Neuronal differentiation and development of synaptic specializations are strongly influenced by cellular interactions. We compared the effects of interaction with distinct autonomic targets on the molecular and biophysical differentiation of 'upstream' neuron-neuron synapses. Contact with cardiac tissue induced expression of nicotinic receptor channels (nAChRs) distinct from those induced by renal tissue in presynaptic autonomic neurons. The kinetics of cholinergic currents at interneuronal synapses are dictated by the peripheral target contacted. Analysis of the nAChR channel subtypes and subunits in individual neurons demonstrated that the profile of transmitter receptors expressed at mature neuron-neuron synapses develops from the convergent influences of input-derived (anterograde) and target-specific (retrograde) signals.
Figures
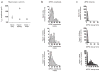
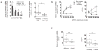


Similar articles
-
Regulation of nAChR subunit gene expression relative to the development of pre- and postsynaptic projections of embryonic chick sympathetic neurons.Dev Biol. 1994 Mar;162(1):56-70. doi: 10.1006/dbio.1994.1066. Dev Biol. 1994. PMID: 8125198
-
Differential modulation of nicotinic acetylcholine receptor subtypes and synaptic transmission in chick sympathetic ganglia by PGE(2).J Neurophysiol. 2001 Jun;85(6):2498-508. doi: 10.1152/jn.2001.85.6.2498. J Neurophysiol. 2001. PMID: 11387396
-
PACAP induces plasticity at autonomic synapses by nAChR-dependent NOS1 activation and AKAP-mediated PKA targeting.Mol Cell Neurosci. 2014 Nov;63:1-12. doi: 10.1016/j.mcn.2014.08.007. Epub 2014 Aug 25. Mol Cell Neurosci. 2014. PMID: 25168001
-
Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system.Brain Res Brain Res Rev. 1999 Nov;30(3):219-35. doi: 10.1016/s0165-0173(99)00016-8. Brain Res Brain Res Rev. 1999. PMID: 10567725 Review.
-
Regulatory mechanisms that govern nicotinic synapse formation in neurons.J Neurobiol. 2002 Dec;53(4):542-55. doi: 10.1002/neu.10112. J Neurobiol. 2002. PMID: 12436419 Review.
Cited by
-
Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons.J Neurosci. 2000 Nov 1;20(21):8069-76. doi: 10.1523/JNEUROSCI.20-21-08069.2000. J Neurosci. 2000. PMID: 11050128 Free PMC article.
-
Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity.J Neurosci. 2002 Sep 15;22(18):8101-9. doi: 10.1523/JNEUROSCI.22-18-08101.2002. J Neurosci. 2002. PMID: 12223564 Free PMC article.
-
Sympathetic tales: subdivisons of the autonomic nervous system and the impact of developmental studies.Neural Dev. 2018 Sep 13;13(1):20. doi: 10.1186/s13064-018-0117-6. Neural Dev. 2018. PMID: 30213267 Free PMC article. Review.
-
The sympathetic nervous system in development and disease.Nat Rev Neurosci. 2021 Nov;22(11):685-702. doi: 10.1038/s41583-021-00523-y. Epub 2021 Oct 1. Nat Rev Neurosci. 2021. PMID: 34599308 Free PMC article. Review.
-
Presynaptic type III neuregulin1-ErbB signaling targets {alpha}7 nicotinic acetylcholine receptors to axons.J Cell Biol. 2008 May 5;181(3):511-21. doi: 10.1083/jcb.200710037. J Cell Biol. 2008. PMID: 18458158 Free PMC article.
References
-
- Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. - PubMed
-
- Yang X, Kuo Y, Devay P, Yu C, Role L. A cysteine-rich isoform of neuregulin controls nicotinic receptor expression in neurons during synaptogenesis. Neuron. 1998;20:255–270. - PubMed
-
- Devay P, Qu X, Role LW. Regulation of nAChR-subunit gene expression relative to the development of pre- and postsynaptic projections of embryonic chick sympathetic neurons. Dev Biol. 1994;162:56–70. - PubMed
-
- Moss BL, Schuetze SM, Role LW. Functional properties and developmental regulation of nicotinic acetylcholine receptors on embryonic chicken sympathetic neurons. Neuron. 1989;3:597–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

