Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development
- PMID: 10449432
- PMCID: PMC408525
- DOI: 10.1172/JCI6629
Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development
Abstract
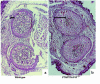
Parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP) bind to and activate the same PTH/PTHrP receptor. Deletion of either the PTHrP gene or the PTH/PTHrP receptor gene leads to acceleration of differentiation of growth plate chondrocytes. To explore further the functional relationships of PTHrP and the PTH/PTHrP receptor, bones of knockout mice were analyzed early in development, and the phenotypes of double-knockout mice were characterized. One early phenotype is shared by both knockouts. Normally, the first chondrocytes to become hypertrophic are located in the centers of long bones; this polarity is greatly diminished in both these knockouts. The PTH/PTHrP receptor-deficient (PTH/PTHrP-R(-/-)) mice exhibited 2 unique phenotypes not shared by the PTHrP(-/-) mice. During intramembranous bone formation in the shafts of long bones, only the PTH/PTHrP-R(-/-) bones exhibit a striking increase in osteoblast number and matrix accumulation. Furthermore, the PTH/PTHrP-R(-/-) mice showed a dramatic decrease in trabecular bone formation in the primary spongiosa and a delay in vascular invasion of the early cartilage model. In the double-homozygous knockout mice, the delay in vascular invasion did not occur. Thus, PTHrP must slow vascular invasion by a mechanism independent of the PTH/PTHrP receptor.
Figures






References
-
- Bringhurst, F.R., Demay, M.B., and Kronenberg, H.M. 1998. Hormones and disorders of mineral metabolism. In Williams textbook of endocrinology. 9th edition. J.D. Wilson, D.W. Foster, H.M. Kronenberg, and P.R. Larsen, editors. W.B. Saunders Co. Philadelphia, PA. 1155–1210.
-
- Yu X, Chandrasekhar S. Parathyroid hormone (PTH 1-34) regulation of rat osteocalcin gene transcription. Endocrinology. 1997;138:3085–3092. - PubMed
-
- Noda M, Toon K, Rodan G. Cyclic AMP-mediated stabilization of osteocalcin mRNA in rat osteoblast-like cells treated with parathyroid hormone. J Biol Chem. 1988;263:18574–18577. - PubMed
-
- Quinn C, et al. Rat collagenase. J Biol Chem. 1990;265:22342–22347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

