Automated quantitative analysis of hepatitis B virus DNA by using the Cobas Amplicor HBV monitor test
- PMID: 10449454
- PMCID: PMC85382
- DOI: 10.1128/JCM.37.9.2793-2797.1999
Automated quantitative analysis of hepatitis B virus DNA by using the Cobas Amplicor HBV monitor test
Abstract
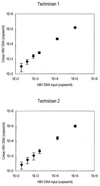
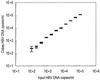
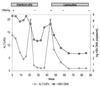
A highly sensitive method of quantitative analysis of hepatitis B virus (HBV) DNA in serum, the Cobas Amplicor HBV Monitor (Cobas-AM) test, was evaluated. Following a manual extraction of viral DNA, amplification, colorimetric detection, and quantitative determination are all automatically performed in the Cobas analyzer. Serially diluted samples with known HBV DNA concentrations were analyzed blindly. All samples with a virus concentration of 400 copies/ml and 83% of samples with a virus concentration of 100 copies/ml could be detected. A linear correlation between input HBV DNA and measured HBV DNA was seen in the range from 100 to 10(5) copies/ml. The mean coefficient of variation was 29.6% for all input levels and 18.9% for HBV DNA concentrations above 400 copies/ml. Samples with an HBV DNA level above 10(9) copies/ml could be reproducibly measured after predilution to 10(-4) or 10(-6) in negative serum; however, the level was underestimated if target DNA after dilution was still above the linear range of the assay. Quantitative results of the Cobas-AM test were interchangeable with measurements by the manual microwell plate version of Amplicor HBV Monitor (MWP-AM); the mean ratio for log Cobas-AM results/log MWP-AM results was 0.97 (standard error of the mean, 0.007) when serum samples from 153 chronic carriers were analyzed. The test should be of value for clinical assessment of chronic carriers and for monitoring the response to antiviral treatment. A limitation is the relatively narrow linear range of the assay, requiring predilution of high-titer (mainly hepatitis B e-antigen-positive) samples.
Figures


References
-
- Allen M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrrell D L, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670–1677. - PubMed
-
- Berger A, Braner J, Doerr H W, Weber B. Quantification of viral load: clinical relevance for human immunodeficiency virus, hepatitis B virus and hepatitis C virus infection. Intervirology. 1998;41:24–34. - PubMed
-
- Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, Koike H, Kobayashi M, Koida I, Arase Y, Saitoh S, Murashima N, Ikeda K, Kumada H. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711–1716. - PubMed
-
- DiDomenico N, Link H, Knobel R, Caratsch T, Weschler W, Loewy Z, Rosenstraus M. COBAS AMPLICORTM: fully automated RNA and DNA amplification and detection system for routing diagnostic PCR. Clin Chem. 1996;42:1915–1923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

