Monitoring of epstein-barr virus DNA load in peripheral blood by quantitative competitive PCR
- PMID: 10449464
- PMCID: PMC85394
- DOI: 10.1128/JCM.37.9.2852-2857.1999
Monitoring of epstein-barr virus DNA load in peripheral blood by quantitative competitive PCR
Abstract
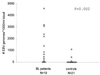
A competitive quantitative PCR (Q-PCR) assay combined with simple silica-based DNA extraction was developed for monitoring of Epstein-Barr virus (EBV) DNA load in unfractionated peripheral blood. The Q-PCR is based on competitive coamplification of a highly conserved 213-bp region of the EBNA-1 open reading frame with an internal standard (IS), added in a known concentration. The IS has the same amplicon length and base composition as the wild-type (WT) EBNA-1 amplicon but differs in 23 internally randomized bases. Competitive coamplification yields two PCR products that are quantified by enzyme immunoassay or by electrochemiluminescence detection, with probes specific for the 23 differing internal nucleotides. The Q-PCR has a sensitivity of 10 copies of either WT or IS plasmid DNA. The Q-PCR was validated by quantification of known amounts of plasmid containing the WT EBNA-1 target. Furthermore, we determined EBV genome copy numbers in different cell lines. For EBV quantification in clinical samples, DNA was isolated from lysed whole blood by silica-affinity purification. Forty-six percent of healthy donor peripheral blood samples were positive by Q-PCR. In most of these samples, viral load was less than 2,000 EBV copies/ml of blood. In peripheral blood samples from two AIDS-related non-Hodgkin's lymphoma patients, elevated EBV loads (up to 120,000 copies/ml) were observed, which decreased upon therapy. In Burkitt's lymphoma patients, up to 4,592,000 EBV genome copies/ml of blood were detected. In conclusion, the EBNA-1-based Q-PCR assay provides a reproducible, accurate, and easy method for studying the relationship between EBV load and clinical parameters.
Figures





Similar articles
-
Toward standardization of Epstein-Barr virus DNA load monitoring: unfractionated whole blood as preferred clinical specimen.J Clin Microbiol. 2001 Apr;39(4):1211-6. doi: 10.1128/JCM.39.4.1211-1216.2001. J Clin Microbiol. 2001. PMID: 11283029 Free PMC article.
-
Comparison of quantitative competitive PCR with LightCycler-based PCR for measuring Epstein-Barr virus DNA load in clinical specimens.J Clin Microbiol. 2002 Nov;40(11):3986-92. doi: 10.1128/JCM.40.11.3986-3992.2002. J Clin Microbiol. 2002. PMID: 12409363 Free PMC article.
-
Oligonucleotides for polymerase chain reaction amplification and hybridization detection of Epstein-Barr virus DNA in clinical specimens.Mol Cell Probes. 1990 Oct;4(5):397-407. doi: 10.1016/0890-8508(90)90030-4. Mol Cell Probes. 1990. PMID: 2177846
-
Epstein Barr viral load monitoring by quantitative PCR in renal transplant patients.New Microbiol. 2003 Apr;26(2):141-9. New Microbiol. 2003. PMID: 12737195
-
High Epstein-Barr virus (EBV) DNA loads in HIV-infected patients: correlation with antiretroviral therapy and quantitative EBV serology.AIDS. 2002 May 3;16(7):993-1001. doi: 10.1097/00002030-200205030-00005. AIDS. 2002. PMID: 11953465
Cited by
-
HLA class I polymorphisms are associated with development of infectious mononucleosis upon primary EBV infection.J Clin Invest. 2007 Oct;117(10):3042-8. doi: 10.1172/JCI32377. J Clin Invest. 2007. PMID: 17909631 Free PMC article.
-
Epstein-Barr Virus-Associated Malignancies and Immune Escape: The Role of the Tumor Microenvironment and Tumor Cell Evasion Strategies.Cancers (Basel). 2021 Oct 16;13(20):5189. doi: 10.3390/cancers13205189. Cancers (Basel). 2021. PMID: 34680337 Free PMC article. Review.
-
Multiple cross displacement amplification combined with nanoparticle-based lateral flow biosensor for rapid and sensitive detection of Epstein-Barr virus.Front Cell Infect Microbiol. 2024 Jan 8;13:1321394. doi: 10.3389/fcimb.2023.1321394. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38259964 Free PMC article.
-
Epstein-Barr Virus DNAemia and post-transplant lymphoproliferative disorder in pediatric solid organ transplant recipients.PLoS One. 2022 Oct 18;17(10):e0269766. doi: 10.1371/journal.pone.0269766. eCollection 2022. PLoS One. 2022. PMID: 36256635 Free PMC article.
-
Epstein-Barr virus can establish infection in the absence of a classical memory B-cell population.J Virol. 2005 Sep;79(17):11128-34. doi: 10.1128/JVI.79.17.11128-11134.2005. J Virol. 2005. PMID: 16103163 Free PMC article.
References
-
- Bai X, Hosler G, Rogers B B, Dawson D B, Scheuermann R H. Quantitative polymerase chain reaction for human herpesvirus diagnosis and measurement of Epstein-Barr virus burden in posttransplant lymphoproliferative disorder. Clin Chem. 1997;43:1843–1849. - PubMed
-
- Bhatia K, Raj A, Guitierrez M I, Judde J G, Spangler G, Venkatesh H, Magrath I T. Variation in the sequence of Epstein-Barr virus nuclear antigen 1 in normal peripheral blood lymphocytes and in Burkitt’s lymphomas. Oncogene. 1996;13:177–181. - PubMed
-
- Drouet E, Brousset P, Fares F, Icart J, Verniol C, Meggetto F, Schlaifer D, Desmorat-Coat H, Rigal-Higuet F, Niveleau A, Delsol G. High Epstein-Barr virus serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin’s disease. J Med Virol. 1999;57:383–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

