Direct evidence for thymic function in adult humans
- PMID: 10449519
- PMCID: PMC2195604
- DOI: 10.1084/jem.190.4.479
Direct evidence for thymic function in adult humans
Abstract
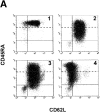
The understanding of human thymic function and evaluation of its contribution to T cell homeostasis are matters of great importance. Here we report the development of a novel assay to quantitate the frequency and diversity of recent thymic emigrants (RTEs) in the peripheral blood of humans. Such cells were defined by the presence of T cell receptor (TCR) rearrangement deletion circles (DCs), episomal byproducts of TCR-beta V(D)J rearrangement. DCs were detected in T cells in the thymus, cord blood, and adult peripheral blood. In the peripheral blood of adults aged 22 to 76 years, their frequency was highest in the CD4(+)CD45RA(+) CD62L(+) subpopulation of naive T cells. TCR DCs were also observed in other subpopulations of peripheral blood T cells, including those with the CD4(+)CD45RO(-)CD62L(+) and CD4(+)CD45RO(+)CD62L(+) phenotypes. RTEs were observed to have more than one Vbeta rearrangement, suggesting that replenishment of the repertoire in the adult is at least oligoclonal. These results demonstrate that the normal adult thymus continues to contribute, even in older individuals, a diverse set of new T cells to the peripheral circulation.
Figures
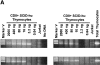
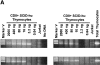





Similar articles
-
T-Cell receptor Vbeta repertoire CDR3 length diversity differs within CD45RA and CD45RO T-cell subsets in healthy and human immunodeficiency virus-infected children.Clin Diagn Lab Immunol. 2000 Nov;7(6):953-9. doi: 10.1128/CDLI.7.6.953-959.2000. Clin Diagn Lab Immunol. 2000. PMID: 11063505 Free PMC article.
-
Fc gamma RII/III and CD2 expression mark distinct subpopulations of immature CD4-CD8- murine thymocytes: in vivo developmental kinetics and T cell receptor beta chain rearrangement status.J Exp Med. 1993 Apr 1;177(4):1079-92. doi: 10.1084/jem.177.4.1079. J Exp Med. 1993. PMID: 8096236 Free PMC article.
-
Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood.J Exp Med. 2002 Mar 18;195(6):789-94. doi: 10.1084/jem.20011756. J Exp Med. 2002. PMID: 11901204 Free PMC article.
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
-
Quantification of T cell receptor rearrangement excision circles to estimate thymic function: an important new tool for endocrine-immune physiology.J Endocrinol. 2003 Mar;176(3):305-11. doi: 10.1677/joe.0.1760305. J Endocrinol. 2003. PMID: 12630915 Review.
Cited by
-
Thymic selection generates a large T cell pool recognizing a self-peptide in humans.J Exp Med. 2002 Feb 18;195(4):485-94. doi: 10.1084/jem.20011658. J Exp Med. 2002. PMID: 11854361 Free PMC article.
-
Immunological effects of interleukin-2 therapy in human immunodeficiency virus-positive subjects.Clin Diagn Lab Immunol. 2001 Jul;8(4):671-7. doi: 10.1128/CDLI.8.4.671-677.2001. Clin Diagn Lab Immunol. 2001. PMID: 11427409 Free PMC article. Review. No abstract available.
-
Multi-omic analyses in immune cell development with lessons learned from T cell development.Front Cell Dev Biol. 2023 Apr 6;11:1163529. doi: 10.3389/fcell.2023.1163529. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37091971 Free PMC article. Review.
-
Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity.JCI Insight. 2017 Sep 21;2(18):e93265. doi: 10.1172/jci.insight.93265. eCollection 2017 Sep 21. JCI Insight. 2017. PMID: 28931755 Free PMC article.
-
Correlates of immune activation marker changes in human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women who use illicit drugs.J Infect Dis. 2003 Jul 15;188(2):209-18. doi: 10.1086/376509. Epub 2003 Jul 1. J Infect Dis. 2003. PMID: 12854075 Free PMC article.
References
-
- Mackall C.L., Hakim F.T., Gress R.E. T-cell regenerationall repertoires are not created equal. Immunol. Today. 1997;18:245–251. - PubMed
-
- McCune J.M. Thymic function in HIV-1 disease. Semin. Immunol. 1997;9:397–404. - PubMed
-
- Kong F., Chen C.H., Cooper M.D. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. - PubMed
-
- Scollay R.G., Butcher E.C., Weissman I.L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 1980;10:210–218. - PubMed
-
- Steinmann G.G. Changes in the human thymus during aging. In: Muller-Hermelink H.K., editor. The Human Thymus—Histopathology and Pathology. Springer-Verlag New York, Inc.; New York, New York: 1986. pp. 43–48.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

