The accessory subunit of mtDNA polymerase shares structural homology with aminoacyl-tRNA synthetases: implications for a dual role as a primer recognition factor and processivity clamp
- PMID: 10449726
- PMCID: PMC22242
- DOI: 10.1073/pnas.96.17.9527
The accessory subunit of mtDNA polymerase shares structural homology with aminoacyl-tRNA synthetases: implications for a dual role as a primer recognition factor and processivity clamp
Abstract
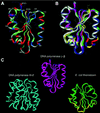
The accessory subunit of the heterodimeric mtDNA polymerase (polgamma) from Drosophila embryos is required to maintain the structural integrity or catalytic efficiency of the holoenzyme. cDNAs for the accessory subunit from Drosophila, man, mouse, and rat have been identified, and comparative sequence alignment reveals that the C-terminal region of about 120 aa is the most conserved. Furthermore, we demonstrate that the accessory subunit of animal polgamma has both sequence and structural similarity with class IIa aminoacyl-tRNA synthetases. Based on sequence similarity and fold recognition followed by homology modeling, we have developed a model of the three-dimensional structure of the C-terminal region of the accessory subunit of polgamma. The model reveals a rare five-stranded beta-sheet surrounded by four alpha-helices with structural homology to the anticodon-binding domain of class IIa aminoacyl-tRNA synthetases. We postulate that the accessory subunit plays a role in the recognition of RNA primers in mtDNA replication, to recruit polgamma to the template-primer junction. A similar role is served by the gamma-complex in Escherichia coli DNA polymerase III, and indeed our accessory subunit model shows structural similarity with the N-terminal domain of the delta' subunit of the gamma-complex. Structural similarity is also found with E. coli thioredoxin, the accessory subunit and processivity factor in bacteriophage T7 DNA polymerase. Thus, we propose that the accessory subunit of polgamma is involved both in primer recognition and in processive DNA strand elongation.
Figures

Similar articles
-
Protein sequences conserved in prokaryotic aminoacyl-tRNA synthetases are important for the activity of the processivity factor of human mitochondrial DNA polymerase.Nucleic Acids Res. 2000 Mar 1;28(5):1237-44. doi: 10.1093/nar/28.5.1237. Nucleic Acids Res. 2000. PMID: 10666468 Free PMC article.
-
The accessory subunit of Xenopus laevis mitochondrial DNA polymerase gamma increases processivity of the catalytic subunit of human DNA polymerase gamma and is related to class II aminoacyl-tRNA synthetases.Mol Cell Biol. 1999 Jun;19(6):4039-46. doi: 10.1128/MCB.19.6.4039. Mol Cell Biol. 1999. PMID: 10330144 Free PMC article.
-
A novel processive mechanism for DNA synthesis revealed by structure, modeling and mutagenesis of the accessory subunit of human mitochondrial DNA polymerase.J Mol Biol. 2006 May 19;358(5):1229-43. doi: 10.1016/j.jmb.2006.02.073. Epub 2006 Mar 15. J Mol Biol. 2006. PMID: 16574152 Free PMC article.
-
Sequence, structure and evolutionary relationships between class 2 aminoacyl-tRNA synthetases: an update.Biochimie. 1993;75(12):1077-81. doi: 10.1016/0300-9084(93)90006-e. Biochimie. 1993. PMID: 8199242 Review.
-
Mutations in DNA polymerase gamma cause error prone DNA synthesis in human mitochondrial disorders.Acta Biochim Pol. 2003;50(1):155-67. Acta Biochim Pol. 2003. PMID: 12673356 Review.
Cited by
-
Protein sequences conserved in prokaryotic aminoacyl-tRNA synthetases are important for the activity of the processivity factor of human mitochondrial DNA polymerase.Nucleic Acids Res. 2000 Mar 1;28(5):1237-44. doi: 10.1093/nar/28.5.1237. Nucleic Acids Res. 2000. PMID: 10666468 Free PMC article.
-
A mechanistic view of human mitochondrial DNA polymerase gamma: providing insight into drug toxicity and mitochondrial disease.Biochim Biophys Acta. 2010 May;1804(5):1213-22. doi: 10.1016/j.bbapap.2010.01.007. Epub 2010 Jan 18. Biochim Biophys Acta. 2010. PMID: 20083238 Free PMC article. Review.
-
Evolution of the metazoan mitochondrial replicase.Genome Biol Evol. 2015 Mar 3;7(4):943-59. doi: 10.1093/gbe/evv042. Genome Biol Evol. 2015. PMID: 25740821 Free PMC article.
-
Mitochondrial DNA maintenance in Drosophila melanogaster.Biosci Rep. 2022 Nov 30;42(11):BSR20211693. doi: 10.1042/BSR20211693. Biosci Rep. 2022. PMID: 36254835 Free PMC article. Review.
-
The accessory subunit of DNA polymerase gamma is essential for mitochondrial DNA maintenance and development in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4483-8. doi: 10.1073/pnas.072664899. Epub 2002 Mar 26. Proc Natl Acad Sci U S A. 2002. PMID: 11917141 Free PMC article.
References
-
- Wernette C M, Kaguni L S. J Biol Chem. 1986;261:14764–14770. - PubMed
-
- Olson M W, Wang Y, Elder R H, Kaguni L S. J Biol Chem. 1995;270:28932–28937. - PubMed
-
- Lewis D L, Farr C L, Wang Y, Lagina A T, III, Kaguni L S. J Biol Chem. 1996;271:23389–23394. - PubMed
-
- Wernette C M, Conway M C, Kaguni L S. Biochemistry. 1988;27:6046–6054. - PubMed
-
- Kaguni L S, Wernette C M, Conway M C, Yang-Cashman P. In: Cancer Cells: Eukaryotic DNA Replication. Kelly T, Stillman B, editors. Vol. 6. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 425–432.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

