Imaging of thermal activation of actomyosin motors
- PMID: 10449739
- PMCID: PMC22255
- DOI: 10.1073/pnas.96.17.9602
Imaging of thermal activation of actomyosin motors
Abstract
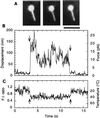
We have developed temperature-pulse microscopy in which the temperature of a microscopic sample is raised reversibly in a square-wave fashion with rise and fall times of several ms, and locally in a region of approximately 10 micrometers in diameter with a temperature gradient up to 2 degrees C/micrometers. Temperature distribution was imaged pixel by pixel by image processing of the fluorescence intensity of rhodamine phalloidin attached to (single) actin filaments. With short pulses, actomyosin motors could be activated above physiological temperatures (higher than 60 degrees C at the peak) before thermally induced protein damage began to occur. When a sliding actin filament was heated to 40-45 degrees C, the sliding velocity reached 30 micrometers/s at 25 mM KCl and 50 micrometers/s at 50 mM KCl, the highest velocities reported for skeletal myosin in usual in vitro assay systems. Both the sliding velocity and force increased by an order of magnitude when heated from 18 degrees C to 40-45 degrees C. Temperature-pulse microscopy is expected to be useful for studies of biomolecules and cells requiring temporal and/or spatial thermal modulation.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

