Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages
- PMID: 10449748
- PMCID: PMC22264
- DOI: 10.1073/pnas.96.17.9648
Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages
Abstract
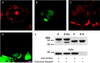
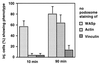
Wiskott-Aldrich syndrome protein (WASp) is a hematopoietic-specific, multidomain protein whose mutation is responsible for the immunodeficiency disorder Wiskott-Aldrich syndrome. WASp contains a binding motif for the Rho GTPase CDC42Hs as well as verprolin/cofilin-like actin-regulatory domains, but no specific actin structure regulated by CDC42Hs-WASp has been identified. We found that WASp colocalizes with CDC42Hs and actin in the core of podosomes, a highly dynamic adhesion structure of human blood-derived macrophages. Microinjection of constitutively active V12CDC42Hs or a constitutively active WASp fragment consisting of the verprolin/cofilin-like domains led to the disassemly of podosomes. Conversely, macrophages from patients expressing truncated forms of WASp completely lacked podosomes. These findings indicate that WASp controls podosome assembly and, in cooperation with CDC42Hs, podosome disassembly in primary human macrophages.
Figures


References
-
- Kirchhausen T. Mol Med Today. 1998;9:300–304. - PubMed
-
- Kenney D M, Cairns L, Remold-O′Donnel E, Peterson J, Rosen F S, Parman R. Blood. 1986;68:1329–1332. - PubMed
-
- Remold-O’Donnell E, Rosen F S, Kenney D M. Blood. 1996;87:2621–2631. - PubMed
-
- Ochs H D. Springer Semin Immunopathol. 1998;19:459–478. - PubMed
-
- Binks M, Jones G E, Brickell P M, Kinnon C, Katz D R, Thrasher A J. Eur J Immunol. 1998;28:3259–3267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

