Role of the Hrp type III protein secretion system in growth of Pseudomonas syringae pv. syringae B728a on host plants in the field
- PMID: 10449783
- PMCID: PMC22299
- DOI: 10.1073/pnas.96.17.9851
Role of the Hrp type III protein secretion system in growth of Pseudomonas syringae pv. syringae B728a on host plants in the field
Abstract
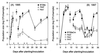
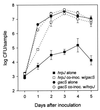
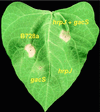
hrp genes are reportedly required for pathogenicity in Pseudomonas syringae pv. syringae (Pss) and other phytopathogenic bacterial species. A subset of these genes encodes a type III secretion system through which virulence factors are thought to be delivered to plant cells. In this study, we sought to better understand the role that hrp genes play in interactions of Pss with its host as they occur naturally under field conditions. Population sizes of hrp mutants with defects in genes that encode components of the Hrp secretion system (DeltahrcC::nptII and hrpJ:: OmegaSpc) and a protein secreted via the system (DeltahrpZ::nptII) were similar to B728a on germinating seeds. However, phyllosphere (i.e., leaf) population sizes of the hrcC and hrpJ secretion mutants, but not the hrpZ mutant, were significantly reduced relative to B728a. Thus, the Hrp type III secretion system, but not HrpZ, plays an important role in enabling Pss to flourish in the phyllosphere, but not the spermosphere. The hrcC and hrpJ mutants caused brown spot lesions on primary leaves at a low frequency when they were inoculated onto seeds at the time of planting. Pathogenic reactions also were found when the hrp secretion mutants were co-infiltrated into bean leaves with a non-lesion-forming gacS mutant of B728a. In both cases, the occurrence of disease was associated with elevated population sizes of the hrp secretion mutants. The role of the Hrp type III secretion system in pathogenicity appears to be largely mediated by its requirement for growth of Pss in the phyllosphere. Without growth, disease does not occur.
Figures


References
-
- Hirano S S, Upper C D. Annu Rev Phytopathol. 1990;28:155–177.
-
- Hirano S S, Rouse D I, Clayton M K, Upper C D. Plant Dis. 1995;79:1085–1093.
-
- Ercolani G L, Hagedorn D J, Kelman A, Rand R E. Phytopathology. 1974;64:1330–1339.
-
- Lindemann J, Arny D C, Upper C D. Phytopathology. 1984;74:1329–1333.
-
- Lindemann J, Arny D C, Upper C D. Phytopathology. 1984;74:1334–1339.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

