Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types
- PMID: 10449786
- PMCID: PMC22302
- DOI: 10.1073/pnas.96.17.9867
Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types
Abstract
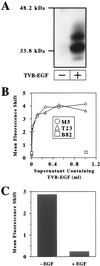
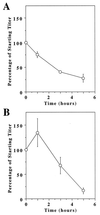
Successful targeting methods represent a major hurdle to the use of retroviral vectors in cell-specific gene-delivery applications. We recently described an approach for retroviral targeting with a retroviral receptor-ligand bridge protein that was bound to the cognate cell-surface ligand receptors before viral challenge. We now report a significant improvement made to this viral targeting method by using a related bridge protein, designated TVB-EGF, comprised of the extracellular domain of the TVB receptor for subgroup B avian leukosis virus fused to epidermal growth factor (EGF). The most important activity of TVB-EGF was that it allowed specific viral entry when preloaded onto virions. Furthermore, virions preloaded with TVB-EGF were thermostable and could be produced directly from virus- packaging cells. These data suggest an approach for targeting retroviral vectors to specific cell types by using virions preloaded with a retroviral receptor-ligand bridge protein and indicate that these types of bridge proteins may be useful reagents for studying the normal mechanism of retroviral entry.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

