Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems
- PMID: 10449789
- PMCID: PMC22305
- DOI: 10.1073/pnas.96.17.9885
Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems
Abstract
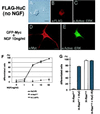
Hu proteins are mammalian embryonic lethal abnormal visual system (ELAV)-like neuronal RNA-binding proteins that contain three RNA recognition motifs. Although Drosophila ELAV is required for the correct differentiation and survival of neurons, the roles played by the Hu genes in the mammalian nervous system remain largely unknown. To explore the in vivo functions of mouse Hu proteins, we overexpressed them in rat pheochromocytoma PC12 cells, where they induced neuronal phenotype in the absence of nerve growth factor. We have characterized the functions of various forms of mHuB and mHuC bearing point mutations or deletions. Mutants of mHuC that had amino acid exchanges in the RNP1 domain of the first or second RNA recognition motifs (RRMs) lost biologic activity as well as RNA-binding activity. In addition, the mutants containing only the third RRM failed to induce the neuronal phenotype in PC12 cells and inhibited the biologic activity of cotransfected wild-type mHuB and mHuC, thus acting as a dominant-negative form. However, these mutants could not suppress the nerve growth factor-induced differentiation of PC12 cells. Further, we misexpressed wild-type and dominant-negative Hu in E9.5 mouse embryos, by using electroporation into the neural tube at the level of the rhombencephalon. mHuB and mHuC induced the ectopic expression of neuronal markers, whereas the dominant-negative forms of mHuB and mHuC suppressed the differentiation of central nervous system motor neurons. From these results, we suggest that Hu proteins are required for neuronal differentiation in the mammalian nervous system.
Figures

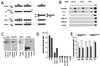

References
-
- Okano H. Dev Growth Differ. 1995;37:619–629. - PubMed
-
- Liu Q, Siomi H, Siomi M C, Fischer U, Zhang Y, Wan L, Dreyfuss G. Cold Spring Harbor Symp Quant Biol. 1996;61:689–697. - PubMed
-
- Grabowski P J. Cell. 1998;92:709–712. - PubMed
-
- Robinow S, Campos A R, Yao K M, White K. Science. 1988;242:1570–1572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

