theta, a novel gamma-aminobutyric acid type A receptor subunit
- PMID: 10449790
- PMCID: PMC22306
- DOI: 10.1073/pnas.96.17.9891
theta, a novel gamma-aminobutyric acid type A receptor subunit
Abstract
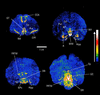
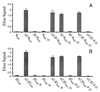
gamma-Aminobutyric acid type A (GABA-A) receptors are a major mediator of inhibitory neurotransmission in the mammalian central nervous system, and the site of action of a number of clinically important drugs. These receptors exist as a family of subtypes with distinct temporal and spatial patterns of expression and distinct properties that presumably underlie a precise role for each subtype. The newest member of this gene family is the theta subunit. The deduced polypeptide sequence is 627 amino acids long and has highest sequence identity (50.5%) with the beta1 subunit. Within the rat striatum, this subunit coassembles with alpha2, beta1, and gamma1, suggesting that gamma-aminobutyric acid type A receptors consisting of arrangements other than alpha beta + gamma, delta, or epsilon do exist. Expression of alpha2beta1gamma1theta in transfected mammalian cells leads to the formation of receptors with a 4-fold decrease in the affinity for gamma-aminobutyric acid compared with alpha2beta1gamma1. This subunit has a unique distribution, with studies so far suggesting significant expression within monoaminergic neurons of both human and monkey brain.
Figures


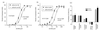
References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

