Synaptically released glutamate reduces gamma-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors
- PMID: 10449797
- PMCID: PMC22313
- DOI: 10.1073/pnas.96.17.9932
Synaptically released glutamate reduces gamma-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors
Abstract
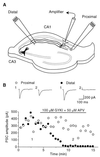
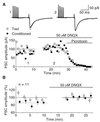
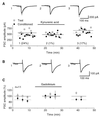
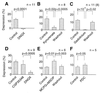
Exogenous application of agonists at the kainate subtype of glutamate receptors has been shown to depress evoked monosynaptic inhibition by gamma-aminobutyric acid (GABA)ergic interneurons in the hippocampus. This observation has led to the hypothesis that synaptic release of endogenous glutamate might have a disinhibitory effect on neuronal circuits, in addition to depolarizing neurons via postsynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate, and N-methyl-D-aspartic acid (NMDA) receptors. It is not known, however, if glutamate released from excitatory neurons has the same kainate receptor-mediated effect on monosynaptic inhibitory transmission as exogenous agonist application. Indeed, the recent demonstration that excitatory synaptic signals elicited in interneurons are partly mediated by kainate receptors suggests that these receptors may have a pro- rather than disinhibitory role. Here, we examine the effect of synaptically released glutamate on monosynaptic inhibitory signaling. In the presence of antagonists to AMPA and NMDA receptors, brief bursts of activity in glutamatergic afferent fibers reduce GABAergic transmission. This depression of inhibition is reversibly abolished by blocking kainate receptors. It persists when GABA(B) receptors are blocked and is enhanced by blocking metabotropic glutamate receptors, possibly explained by presynaptic regulation of glutamate release from excitatory afferents by metabotropic autoreceptors. We conclude that the net kainate receptor-mediated effect of synaptically released glutamate is to reduce monosynaptic inhibition. Since this form of disinhibition may contribute to seizure initiation, kainate receptors may constitute an important target for anticonvulsant drug development.
Figures
Similar articles
-
Regulation of spontaneous inhibitory synaptic transmission by endogenous glutamate via non-NMDA receptors in cultured rat hippocampal neurons.Neuropharmacology. 2001 May;40(6):737-48. doi: 10.1016/s0028-3908(00)00213-6. Neuropharmacology. 2001. PMID: 11369028
-
Presynaptic kainate receptors regulate spinal sensory transmission.J Neurosci. 2001 Jan 1;21(1):59-66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. J Neurosci. 2001. PMID: 11150320 Free PMC article.
-
Glutamatergic modulation of GABAergic signaling among hippocampal interneurons: novel mechanisms regulating hippocampal excitability.Epilepsia. 2002;43 Suppl 5:174-8. doi: 10.1046/j.1528-1157.43.s.5.12.x. Epilepsia. 2002. PMID: 12121316
-
[Kainate receptors. Their function in the regulation of GABAergic synaptic transmission in the hippocampus].Rev Neurol. 2003 May 1-15;36(9):852-9. Rev Neurol. 2003. PMID: 12717674 Review. Spanish.
-
In the developing hippocampus kainate receptors control the release of GABA from mossy fiber terminals via a metabotropic type of action.Adv Exp Med Biol. 2011;717:11-26. doi: 10.1007/978-1-4419-9557-5_2. Adv Exp Med Biol. 2011. PMID: 21713663 Review.
Cited by
-
Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus.Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12917-22. doi: 10.1073/pnas.96.22.12917. Proc Natl Acad Sci U S A. 1999. PMID: 10536023 Free PMC article.
-
Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk.Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):2151-5. doi: 10.1073/pnas.0308408100. Epub 2004 Feb 6. Proc Natl Acad Sci U S A. 2004. PMID: 14766975 Free PMC article.
-
Synaptic kainate receptors in CA1 interneurons gate the threshold of theta-frequency-induced long-term potentiation.J Neurosci. 2012 Dec 12;32(50):18215-26. doi: 10.1523/JNEUROSCI.2327-12.2012. J Neurosci. 2012. PMID: 23238735 Free PMC article.
-
Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain.J Physiol. 2016 Oct 1;594(19):5471-90. doi: 10.1113/JP271764. Epub 2016 May 12. J Physiol. 2016. PMID: 26918438 Free PMC article. Review.
-
Control of puberty by excitatory amino acid neurotransmitters and its clinical implications.Endocrine. 2005 Dec;28(3):281-6. doi: 10.1385/ENDO:28:3:281. Endocrine. 2005. PMID: 16388117 Review.
References
-
- Clarke V R, Ballyk B A, Hoo K H, Mandelzys A, Pellizzari A, Bath C P, Thomas J, Sharpe E F, Davies C H, Ornstein P L, et al. Nature (London) 1997;389:599–603. - PubMed
-
- Rodriguez-Moreno A, Herreras O, Lerma J. Neuron. 1997;19:893–901. - PubMed
-
- Lerma J. Neuron. 1997;19:1155–1158. - PubMed
-
- Cossart R, Esclapez M, Hirsch J C, Bernard C, Ben-Ari Y. Nat Neurosci. 1998;1:470–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

