Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila
- PMID: 10454375
- PMCID: PMC311269
Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila
Abstract
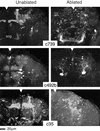
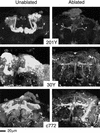
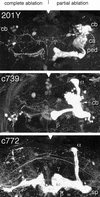
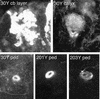
Paired brain centers known as mushroom bodies are key features of the circuitry for insect associative learning, especially when evoked by olfactory cues. Mushroom bodies have an embryonic origin, and unlike most other brain structures they exhibit developmental continuity, being prominent components of both the larval and the adult CNS. Here, we use cell-type-specific markers, provided by the P[GAL4] enhancer trap system, to follow specific subsets of mushroom body intrinsic and extrinsic neurons from the larval to the adult stage. We find marked structural differences between the larval and adult mushroom bodies, arising as the consequence of large-scale reorganization during metamorphosis. Extensive, though incomplete, degradation of the larval structure is followed by establishment of adult specific alpha and beta lobes. Kenyon cells of embryonic origin, by contrast, were found to project selectively to the adult gamma lobe. We propose that the gamma lobe stores information of relevance to both developmental stages, whereas the alpha and beta lobes have uniquely adult roles.
Figures




References
-
- Aceves-Pina AE, Quinn WG. Learning in normal and mutant Drosophila larvae. Science. 1979;206:93–96. - PubMed
-
- Armstrong JD, Kaiser K. The study of Drosophila brain development. In: Houdebine LM, editor. Transgenic animals—Generation and use. Harwood Academic Publishers; 1997. pp. 365–370.
-
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Brotz T M, Bochenek B, Aronstein K, ffrench-Constant R H, Borst A. γ aminobutyric acid receptor distribution in the mushroom bodies of a fly (Calliphora erythrocephala): A functional subdivision of Kenyon cells? J Comp Neurol. 1996;383:42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
