Open randomised trial of intermittent very low energy diet together with nicotine gum for stopping smoking in women who gained weight in previous attempts to quit
- PMID: 10454403
- PMCID: PMC28202
- DOI: 10.1136/bmj.319.7208.490
Open randomised trial of intermittent very low energy diet together with nicotine gum for stopping smoking in women who gained weight in previous attempts to quit
Abstract
Objective: To determine whether attempts to prevent weight gain will increase success rates for stopping smoking.
Design: 16 week, open, randomised study with 1 year follow up.
Setting: Obesity unit.
Subjects: 287 female smokers who had quit smoking before but started again because of weight concerns.
Intervention: Combination of a standard smoking cessation programme with nicotine gum and a behavioural weight control programme including a very low energy diet. A control group was treated with the identical programme but without the diet.
Main outcome measure: Sustained cessation of smoking.
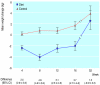
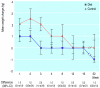
Results: After 16 weeks, 68/137 (50%) women had stopped smoking in the diet group versus 53/150 (35%) in the control group (P=0.01). Among these women, weight fell by mean 2.1 (95% confidence interval 2.9 to 1.3) kg in the diet group but increased by 1.6 (0.9 to 2.3) kg in the control group (P<0.001). After 1 year the success rates in the diet and control groups were 38/137 (28%) and 24/150 (16%) respectively (P<0.05), but there was no statistical difference in weight gain.
Conclusions: Combining the smoking cessation programme with an intervention to control weight helped women to stop smoking and control weight.
Figures
Comment in
-
Results are unlikely to be as good in routine practice.BMJ. 1999 Aug 21;319(7208):494. doi: 10.1136/bmj.319.7208.494. BMJ. 1999. PMID: 10454404 Free PMC article. No abstract available.
Comment on
-
Polymorphism in high density lipoprotein paraoxonase gene and risk of acute myocardial infarction in men: prospective nested case-control study.BMJ. 1999 Aug 21;319(7208):487-9; discussion 490. doi: 10.1136/bmj.319.7208.487. BMJ. 1999. PMID: 10454401 Free PMC article. No abstract available.
References
-
- Silagy C, Mant D, Fowler G, Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet. 1994;343:139–142. - PubMed
-
- US Public Health Service. The health benefits of smoking cessation. A report of the surgeon general. Washington, DC: Department of Health and Human Services; 1990. pp. 469–515. . (DHHS publication No (CDC) 90-416).
-
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. - PubMed
-
- Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;33:1165–1170. - PubMed
-
- Klesges RC, Klesges LM, Meyers AW. Relationship of smoking status, energy balance, and body weight: analysis of the second national health and nutrition examination survey. J Consult Clin Psychol. 1991;59:899–905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
